Sequence of Events During Synaptic Transmission
A brief summary of the basic sequence of events that occurs during synaptic transmission at a typical synapse is as follows:

Some of these events are shown graphically in Figure 1. An action potential arriving at the terminal of a presynaptic axon causes voltage-gated Ca2+ channels at the active zone to open. The influx of Ca2+ ions through these channels produces a high concentration of Ca2+ ions near the active zone, which causes the vesicles containing neurotransmitter to fuse with the presynaptic cell membrane and release their contents into the synaptic cleft (exocytosis), the neurotransmitter molecules then diffuse across the synaptic cleft and bind to specific receptors on the post-synaptic membrane. These receptors cause ion channels to open, thereby changing the membrane conductance and membrane potential of the postsynaptic cell.
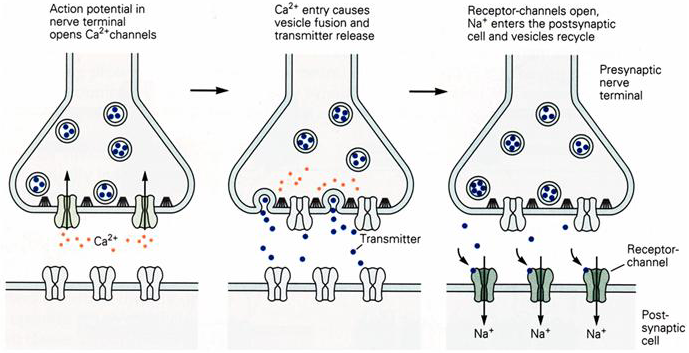
Figure 1 Sequence of events during synaptic transmission.
Each of these steps is described in further detail below:
1. Action potential travels down axon.
The action potential travels down the axon and invades the nerve terminal essentially as described in Chapter 4.
2. Action potential invades the nerve terminal causing a depolarization.
Voltage-gated Na+ and K+ channels are also found in the nerve terminal so that when the action potential reaches the nerve terminal the membrane potential in the terminal is rapidly depolarized.
3. Depolarization opens voltage-gated Ca2+ channels.
There are also voltage-gated Ca2+ channels in the nerve terminal, which are not present in the axon. The voltage-gated Ca2+channels are activated during an action potential by depolarization, much like voltage-gated Na+ and K+ channels, but do not contribute greatly to current flow during the action potential because they are significantly less abundant than the other channels. They are critically important because they mediate the influx of Ca2+ ions into the nerve terminal.
4. Influx of Ca2+ ions through the Ca2+ channels increases the Ca2+ concentration inside the nerve terminal.
The coupling between nerve terminal depolarization and neurotransmitter release is not direct. Calcium ions act as second messengers triggering the release of neurotransmitter. If Ca2+ ions are removed from the extracellular fluid, then the action potential can no longer elicit the release of neurotransmitter. Neurotransmitter release is absolutely dependent upon the influx of Ca2+ ions into the presynaptic cell.
Calcium ions can act as a second messenger because they are the exception to the general rule, that ion fluxes across the cell membrane induced by electrical activity do not have a significant effect on intracellular ion concentrations. This difference is due to the fact that intracellular Ca2+ ion concentrations are extremely low inside the cell ([Ca2+]i = 100 nM) relative to external Ca2+ ion concentrations ([Ca2+]o = 2 mM), so that the influx of relatively few Ca2+ ions can produce a significant, if transient, increase in Ca2+ ion concentration close to channels in the cell membrane.
The influx of Ca2+ ions converts an electrical signal into a biochemical signal, which triggers all the subsequent events inside the nerve terminal.
5. Increase in internal Ca2+ concentration promotes the fusion of synaptic vesicles with the cell membrane.
The rise in internal Ca2+ levels triggers neurotransmitter release by promoting the fusion of synaptic vesicles with the plasma membrane. It is possible to visualize the vesicles, using electron microscopy. A subset of the vesicles are docked or tethered to a structure known as the release site or active zone. It is the vesicles located at the active zone that are the ones released following an influx of Ca2+ ions.
Also clustered at these release sites are the voltage-gated Ca2+ channels. Influx of Ca2+ produces a large increase in Ca2+ concentrations immediately beneath the cell membrane at the release sites. The increase in Ca2+ concentration is much larger near these channels than in the bulk of the cytoplasm.
6. Fusion of synaptic vesicles releases neurotransmitter into synaptic cleft.
The regulation of vesicular fusion and its responsiveness to changes in Ca2+ ion concentration is complex and requires the coordinated action of a large number of different proteins. The process of vesicle fusion is known as exocytosis. Exocytosis is evolutionary ancient, dating back at least to stem eukaryotes. It is necessary in eukaryotes to move material between different membrane delimited compartments within the cell. This process is known as constitutive exocytosis. Synaptic vesicle release is an example of regulated exocytosis, where exocytosis will only proceed in response to a specific signal, calcium ions.
The main protein components necessary for synaptic vesicle fusion are shown in Figure 2.
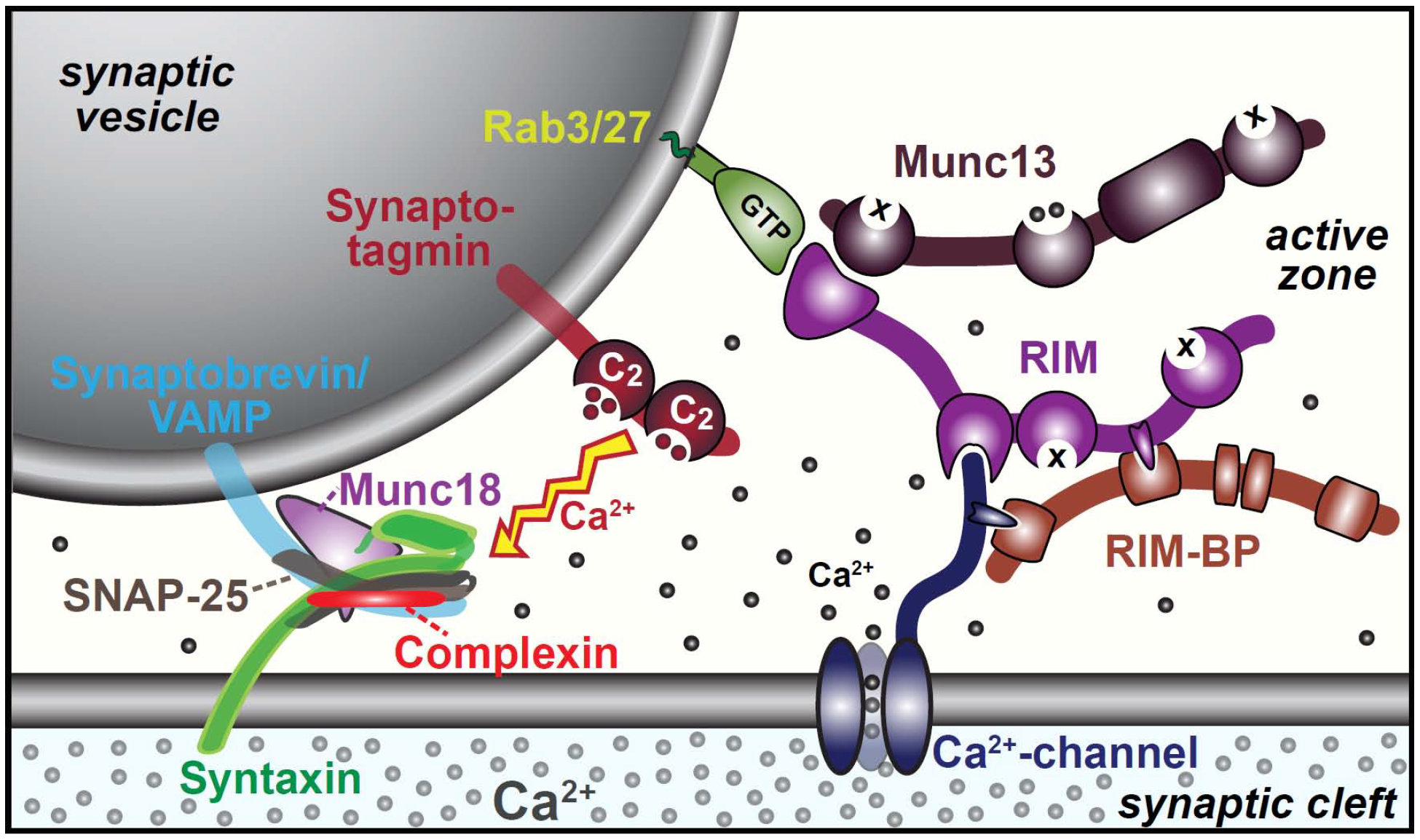
Figure 2 Main protein components that mediate synaptic vesicle release.
There are three basic functions that these proteins perform in order to facilitate rapid and regulated synaptic vesicle release.
1. Vesicle tethering
2. Calcium triggering of fusion
3. Vesicle fusion
Each of these processes is mediated by a different set of proteins.
Table 1 Proteins Involved in Synaptic Vesicle Fusion
The vesicle tethering proteins create a nanodomain by bringing the calcium channel into very close proximity with the calcium sensing protein synaptotagmin and the vesicle fusion proteins. This organization dramatically increases both the speed and specificity of calcium signaling. Large changes in free calcium ion concentration occur only in the immediate vicinity of the channel if it only opens briefly and the short diffusion distances reduce the time taken to complete the signaling reaction.
Synaptotagmin is the protein that binds Ca2+ ions and then triggers the vesicle fusion process. This second step requires cooperation with the protein complexin.
Vesicle fusion requires the generation of force to overcome the electrostatic repulsion between the vesicle and cell membranes. This force is generated by the zippering of the three SNARE proteins synaptobrevin, syntaxin and SNAP-25.
Subsequent recycling of the entangled SNARE proteins requires the NSF protein.
7. Neurotransmitter binds to neurotransmitter receptors in cell membrane of post-synaptic cell.
When the vesicle fuses, the neurotransmitter molecules inside the vesicle are released into the synaptic cleft and begin to diffuse across the cleft to the post-synaptic cell where a fraction of the molecules bind to neurotransmitter receptors.
8. Binding of neurotransmitter to the receptor induces a conformational change in the receptor that opens an ion channel.
The neurotransmitter receptor combines two functions, it has a binding site for neurotransmitter, and it functions as an ion channel. Binding of neurotransmitter opens a gate in the channel, which then allows ions to flow through the pore of the channel.
9. Opening of ion channels produces a synaptic current in the postsynaptic cell.
The opening of individual agonist-activated channels occurs almost simultaneously (Figure 3) because the neurotransmitter is released from the nerve terminal within a relatively brief time window. The channels remain open for random time intervals. Since processes with random lifetimes decay exponentially, the sum of the individual currents has an exponential decay (Figure 3). This current is known as the excitatory postsynaptic current (epsc).
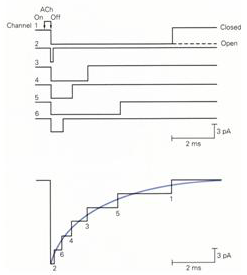
Figure 3 Individual agonist-activated channels open almost simultaneously following neurotransmitter release and then close at random intervals (top panel). The sum of individual currents results in a postsynaptic current that decays exponentially (bottom panel).
10. The synaptic current produces a change in the membrane potential of the postsynaptic cell, possibly triggering an action potential.
An excitatory postsynaptic current produces a depolarization in the post-synaptic cell. This depolarization is known as an excitatory post-synaptic potential (epsp). The epsp is called excitatory because it tends to drive the membrane potential towards the threshold for action potential generation. The membrane potential change (epsp) has a slower time course than the underlying current (epsc) because the membrane capacitance takes a certain amount of time to charge up and then discharge (Figure 4).
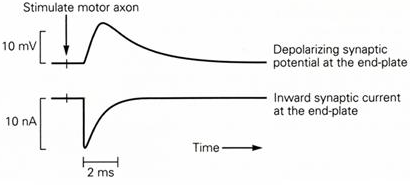
Figure 4 Time course of an epsp and epsc. Note that the epsc is significantly faster than the epsp due to the capacitance of the membrane in the postsynaptic cell.
If the epsp is large enough to reach threshold, Na+ channels begin to open and an action potential is generated by a positive feed-back cycle rapidly opening more Na+ channels.
11. Neurotransmitter is removed from the synaptic cleft.
Once the neurotransmitter is released, it is important to remove it quickly in order to reset the system ready for the next action potential. Typically, neurotransmitter is removed by enzyme action, reuptake, diffusion, or some combination of these mechanisms.
As an example, acetylcholine at the NMJ is quickly inactivated by the enzyme acetylcholinesterase. This enzyme is located in the synaptic cleft. It splits acetylcholine into acetate and choline, neither of which can activate the AChR by themselves. Because of the rapid action of the acetylcholinesterase enzyme, the pulse of acetylcholine in the synaptic cleft following a presynaptic action potential is quite brief.
12. Components of the synaptic vesicles are recycled.
The nerve terminal is quite small and it is a relatively long way from the cell body. As a consequence, its resources are easily depleted. To minimize this problem a considerable amount of recycling occurs at the nerve terminal.
At the NMJ following the enzymatic degradation of ACh much of the choline is then taken back up into the synaptic terminal by active transport. The choline is then converted into ACh by the intracellular enzyme choline acetyltransferase. Neurotransmitters such as glutamate and GABA are also transported back into the nerve terminal by specific neurotransmitter transporters that use the gradient of Na+ ions across the cell membrane as an energy source.
Neurotransmitter is then packaged back into the vesicles by specific vesicular transporters that use a H+ gradient across the vesicle membrane as a source of energy. The proton gradient is generated by an ATP-dependent proton pump (V-ATPase).
The synaptic vesicle membrane and SNARE proteins are also recycled by a process known as endocytosis. After fusing with the plasma membrane to release neurotransmitter the membrane of the vesicle is reabsorbed into clathrin coated pits and ultimately recycled to make new vesicles.
Synaptic Delay
All these processes take some time to complete. The time taken between an action potential arriving in the presynaptic nerve and the initiation of the post-synaptic action potential is known as synaptic delay (Figure 5). There are multiple sources for synaptic delay. There is a delay associated with the activation of the Ca2+ channels, which have slower kinetics than the Na+ channels. Other processes, including exocytosis, diffusion, and activation of neurotransmitter receptors take time, as does charging of membrane capacitance until the voltage reaches threshold to activate Na+ channels. Typical values for synaptic delay are 1-2 ms. Synaptic transmission takes significantly longer than if the two cells were electrically connected so that electrical excitation passed directly from one cell to the other.
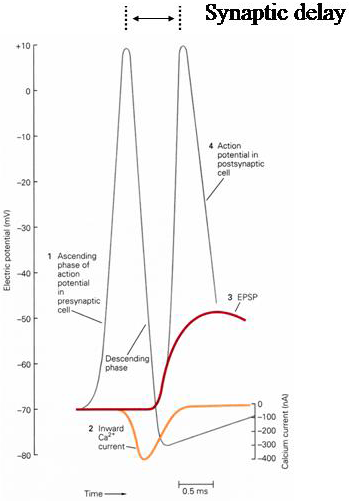
Figure 5 Synaptic delay. Note the delay before the activation of the Ca2+ channels, which turn on during the falling phase of the action potential.