Ion Pumps
Most of the solutes distributed across the cell membrane are not in equilibrium. In particular, several inorganic ions have steep distribution gradients across the cell membrane. As a consequence, energy must be expended in order to maintain those transmembrane concentration gradients. The source of energy is chemical potential energy in the form of ATP. If the cell is poisoned so that ATP is no longer produced, then the transmembrane ion gradients dissipate and the cell dies.
The membrane proteins that move ions against their concentration gradients are known as ion pumps. There are two main families of ion pumps, the P-type ATPase gene family and the V-ATPase gene family.
P-type ATPase Ion Pumps
These ion pumps all have an ATPase activity which auto-catalyzes phosphorylation of the pump. A cycle of phosphorylation and subsequent dephosphorylation drives the cycle of conformational change responsible for ion transport. The name, ‘P-type’, refers to this autophosphorylation property.
The three main types of ion pumps in this gene family that are expressed in vertebrates are listed in Table 1.
Table 1 P-type ATPase ion pumps.
| Pump | Function |
|---|---|
| Na,K-ATPase | maintains the Na+, K+ ion gradients across the cell membrane |
| Ca-ATPase | maintains the very low intracellular Ca2+ ion concentration |
| H,K-ATPase | acid secretion in stomach and kidneys |
Na,K-ATPase
An argument can be made that the Na,K-ATPase pump is the most important protein in the cell because, in most cells, it is the single largest consumer of cellular energy. Surprisingly, the maintenance of ion gradients is the most energetically demanding process within many cells — more expensive than many other apparently more sophisticated functions, such as protein synthesis. This is particularly true in neurons where maintenance of ion gradients becomes very metabolically expensive because of the constant electrical activity and associated ion fluxes.
The Na,K-ATPase pump actively pumps Na+ ions out of the cell, against their concentration gradient. It simultaneously pumps K+ ions into the cell, also against their concentration gradient. The metabolic energy for this activity is provided by the hydrolysis of ATP to ADP. Two K+ ions from outside the cell are exchanged for three Na+ ions from inside the cell during one cycle of the pump which results in the hydrolysis of one ATP molecule (Figure 1).
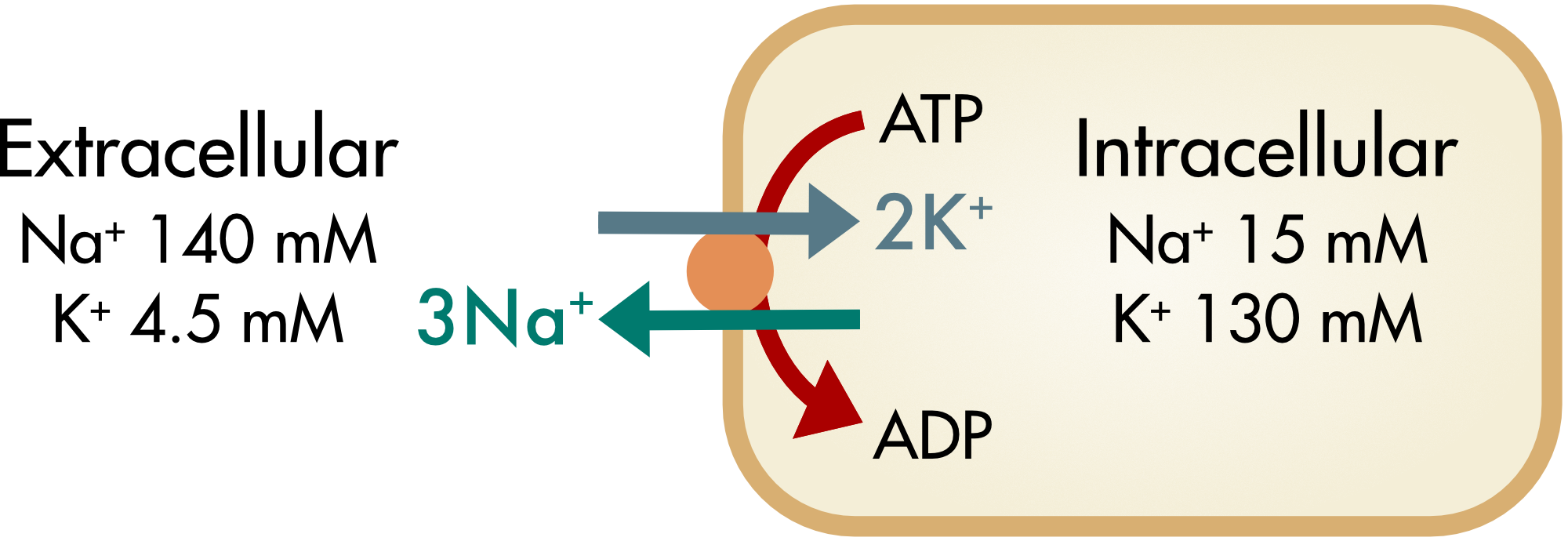
Figure 1 Movement of sodium and potassium ions during one cycle of the Na,K-ATPase pump. With each cycle three sodium ions move out of the cell and two potassium ions move in. As a consequence, one net positive charge moves out of the cell creating a small outward current.
The pump is known as an electrogenic pump because there is an associated electrical current, the net movement of one positive ion out of the cell for every cycle of the pump. This current is generally small relative to most other ion currents in electrically excitable cells. The Na,K-ATPase is present in the plasma membranes of all animal cells. It is responsible for the fact that all animal cells have a relatively high intracellular concentration of potassium ions and a low intracellular concentration of sodium ions.
Ion Gradients as Sources of Cellular Energy
The generation of ion gradients by the Na,K-ATPase pumps is one way in which the cell can convert chemical potential energy, stored in the form of ATP, into another form of chemical potential energy, in this case a concentration gradient of ions. The gradient of ions acts as a source of energy that can be used for other cellular functions such as secondary active transport.
The generation of ion gradients can also convert chemical potential energy into electric potential energy. The ion gradients created by the pumps allow the generation of an electric potential across the cell membrane, known as the membrane potential.
Na,K-ATPase Function
The basic mechanism of the Na,K-ATPase pump is illustrated in Figure 2. The pump has a channel that crosses the membrane. This channel has two gates, on either side of ion binding sites within the channel. The pump can be in one of two main states, known as the E1 and E2 states. In the E1 state, the ion binding sites within the pore of the pump have a high affinity for sodium ions, the outer gate is closed, and the inner gate is open. Autophosphorylation of the E1 state (E1P) is coupled to the binding of three sodium ions to specific sites within the channel. Autophosphorylation produces large structural rearrangements that result in a transition to the E2 state. During this transition the ions are transiently enclosed by gates at either end of the channel. In the E2 state the ion binding sites have reduced affinity for the sodium ions and the outer gate is now open. The sodium ions diffuse out of the channel. Following binding of two potassium ions to two ion binding sites within the pore that now have high affinity for potassium ions, the pump is auto-dephosphorylated. Dephosphorylation is mediated by direct hydrolysis with a water molecule. The inorganic phosphate (Pi) initially remains trapped within the ATP binding pocket. It is released during the transition back to the E1 state, followed by binding of a new ATP molecule.
Figure 2 Animation of the Na,K-ATPase pump cycle. Step through the animation by clicking the ‘step’ button. The reaction scheme on the right indicates the state of the pump during the cycle. There are two gates that enclose the ion binding sites within the channel. The outer gate is closed in the E1 state and the inner gate is closed in the E2 state. The green circles represent potassium ions and the red circles represent sodium ions. The “-P” symbol represents phosphorylation of the pump and “Pi” is the inorganic phosphate released after dephophorylation.
The opening and closing of the gates require large changes in the conformation of the protein and is relatively slow. This limits the cycle time of the pump to frequencies of a hundred times per second. As a consequence, the rate of ion transport by this pump, or any pump, is vastly slower than the rate of ion movement through typical ion channels.
The pump can be poisoned or inhibited with the drug ouabain, which ultimately results in the death of the cell. Ouabain was originally isolated from plants in Africa and was used to poison arrow tips. Surprisingly, similar compounds are used in modern medicine to partially block the pump in the treatment of some forms of congestive heart failure.
Another toxin, palytoxin, binds to the Na,K-ATPase causing the inner and outer gates to open simultaneously thereby turning it into a cation channel. A single palytoxin molecule bound to one Na,K-ATPase molecule is sufficient to dissipate a cell's ionic gradient and kill the cell. The action of this drug illustrates a key point—closing of both gates of the pump is an absolutely essential step before opening either gate. If both gates were open for only 0.001% of the pump cycle this would be sufficient to nullify the action of the pump because of the vast differences in the rate of ion movement through pumps and channels.
Ca-ATPase
There are two closely related types of calcium pumps, those found in the plasma membrane, known as PMCA, (Plasma Membrane Ca2+ ATPase) and those found in the sarcoplasmic and endoplasmic reticulum, known as SERCA (Sarco/Endoplasmic Reticulum Ca2+ ATPase). The concentration of Ca2+ ions in the cytoplasm is very low compared with the extracellular Ca2+ ion concentration, in large part due to active pumping of Ca2+ ions out of the cell or into membrane bound organelles within the cell. There is an approximately 1:10,000 gradient in Ca2+ ion concentration gradient between the interior of the cell and the extracellular fluid.
The calcium pumps function similarly to the Na,K-ATPase. They pump calcium out of the cytoplasm and transport protons as counterions into the cytoplasm.
H,K-ATPase
This pump is the closest relative of the Na,K-ATPase and functions similarly. It pumps two protons, analogous to the sodium ions, and two counterions, which are potassium ions, similarly to the Na,K-ATPase. The third ion binding site is non-functional in this pump. This pump has a much more limited distribution in the body, being primarily involved in acid secretion by specialized cells in the stomach and kidneys.
Structure of Ion Pumps
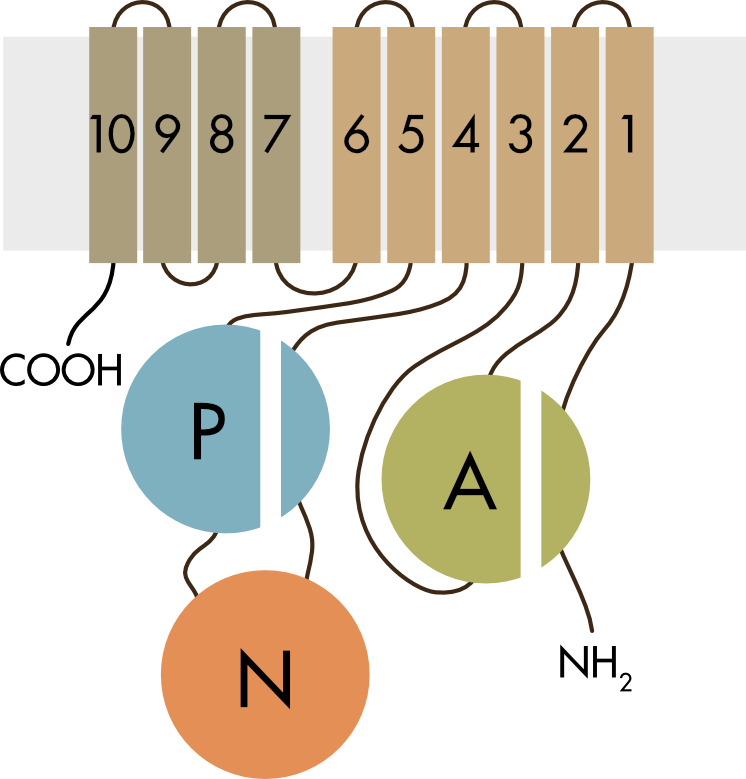
Figure 3 Topology of the alpha subunit of the Na,K-ATPase.
The mammalian P-type pumps have ten transmembrane helices (M1–10) and three cytoplasmic domains: a phosphorylation domain (P-domain), a nucleotide-binding domain (N-domain) and an actuator domain (A-domain). The ion binding sites within the membrane spanning domains and the site of ATP binding, phosphorylation and hydrolysis are separated by more than 50 Å within the molecule.
The detailed structure of the Ca-ATPase pump has been solved. It is a large protein and has ten membrane spanning domains (Figure 4). There are two binding sites for Ca2+ ions within the membrane and it has a very large intracellular domain, which contains the ATPase enzyme that hydrolyzes ATP. The pump undergoes large rearrangements upon phosphorylation and dephosphorylation of the ATPase site (Figure 4). This results in the rearrangement of the alpha helices in the membrane so that the Ca2+ binding sites are moved from facing the intracellular region of the membrane to facing the extracellular region and also causes a reduction of the affinity for Ca2+ ion binding so that the ions are released into the extracellular fluid or the interior of membrane bound organelles.
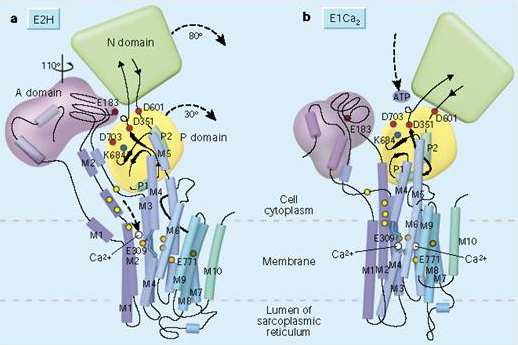
Figure 4 Conformational changes of the Ca-ATPase before and after phosphorylation.
This requirement for a large conformational change limits the rate at which ion pumps can move ions across the membrane. In general, the pumps are continuously active in order to keep up with the flux of ions through the membrane’s ion channels, which allow ions to move very rapidly down their ion concentration gradients. In most cells the ion channels turn on for only brief periods of time in order to limit the amount of work required of the pumps. An exception to this is found in cardiac myocytes, where the Ca2+ pumps have to return Ca2+ ions back to the lumen of the sarcoplasmic reticulum after it has escaped through Ca2+ channels that remain open for the duration of the cardiac contraction. In this case, very high concentrations of the Ca-ATPase pump are required in the SR membrane in order to keep up with the calcium release and this protein makes up a large fraction of the total membrane protein in cardiac cells. This is one reason why we are so vulnerable to ischemia during a heart attack. When blood flow stops even for a short period of time there can be significant damage to the cardiac muscle because it fails to meet the energy demands of the Ca-ATPase pump.
Regulation of P-type ATPases
The activity of the P-type ATPases is regulated in order to optimize energy utilization, since they are major consumers of ATP, and to optimize resting ion concentrations. The activity of the pumps can be regulated by a variety of mechanisms. For the Na,K-ATPase and Ca-ATPase regulation is mediated, in large part, by auxiliary proteins that bind to the pumps.