Cell Membrane
The role of the cell membrane in distinguishing the intracellular fluid from the extracellular fluid is made possible by the fact that it is such an effective barrier to the transport of ions and polar molecules.
Lipid Bilayer Structure
Cell membranes are composed of lipids and proteins. The predominant lipids in the cell membrane are phospholipids. Phospholipids have two distinct regions (Figure 1). A polar region that is hydrophilic (water loving) that interacts with water molecules and a nonpolar region that is hydrophobic (water hating). Molecules that have a mixed chemical nature like this are known as amphipathic molecules.
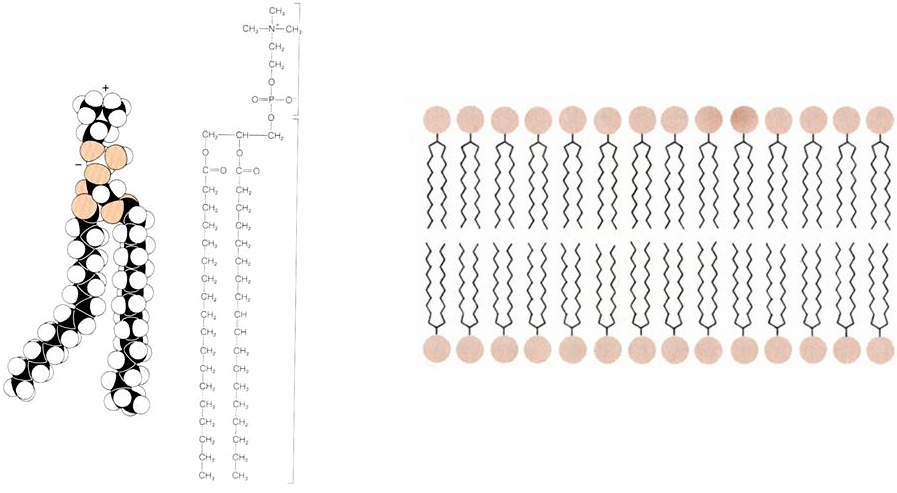
Figure 1 (Left panel) Structure of a phospholipid molecule. Note the charged head of the molecule and the two long hydrophobic tails. (right panel) Arrangement of phospholipids in a lipid bilayer, with the heads pointing out to the aqueous solution and the hydrophobic heads sequestered in the interior of the membrane.
The lipids assemble into a lipid bilayer (Figure 1), which is the lowest energy arrangement for phospholipid molecules. In this arrangement the hydrophobic tails point in towards the center of the bilayer, minimizing their interaction with water molecules. The hydrophilic heads interact with the water molecules surrounding the membrane.
Although the lipid bilayer is very thin, it is a very effective barrier to the diffusion of many biologically important molecules. The interior of the lipid bilayer functions like a very thin layer of oil presenting an almost impermeable barrier to the diffusion of polar or charged molecules. In contrast, hydrophobic molecules can pass easily because they can dissolve into the hydrophobic core of the lipid bilayer.
Charged molecules like ions are stable in a polarizable medium such as water. It is essentially impossible for a charged molecule to cross the lipid bilayer because the core of the bilayer does not readily polarize. In electrical terms, the hydrophobic core has a low relative permittivity (dielectric constant). Ions will not partition into the bilayer from the aqueous phase and cannot normally cross the membrane.
If the cell membrane was composed only of a lipid bilayer only hydrophobic molecules could enter and leave the cell, which would greatly limit the function of the cell. Real cell membranes also contain proteins, and a major function of these proteins is to facilitate the movement of ions and polar molecules across the membrane.
Cell Membrane Structure
Current ideas about how the cell membrane functions originate with the fluid mosaic model of Singer and Nicolson. In their model a class of proteins, known as integral membrane proteins, are sequestered within the lipid bilayer, something like icebergs floating in a lipid sea (Figure 2). These proteins have amphipathic properties, meaning that they have both nonpolar portions, which are buried in the hydrocarbon core of the bilayer, and polar or charged portions, which protrude from the bilayer to form a hydrophilic surface that interacts with the aqueous phase. Typically, charged residues on the surface of the protein are only found in those surface regions that interact with the aqueous solution.
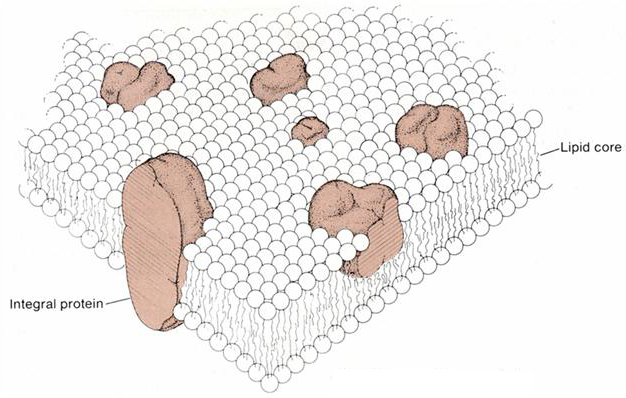
Figure 2 Fluid mosaic model of the cell membrane, showing the integral proteins and the lipid bilayer.
In addition to integral membrane proteins there are also peripheral membrane proteins, which are typically located on the inner surface of the cell membrane (Figure 3). These are often linked to cytoskeletal proteins, which control cell shape and motility. In the original model, the integral membrane proteins were considered to move freely within the plane of the lipid bilayer. In fact, the majority of proteins are tethered to a dense network of other proteins, forming large protein complexes.
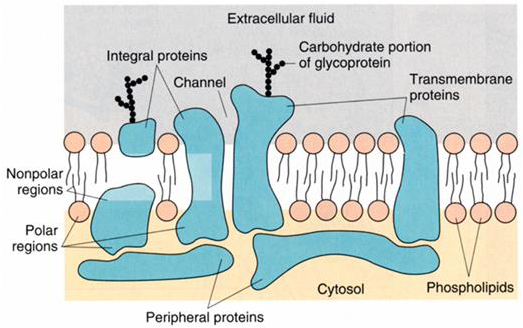
Figure 3 Multiple types of proteins contribute to the formation of the cell membrane.
Typically, the transmembrane proteins (those that cross the cell membrane to the extracellular surface) have covalently linked carbohydrates on their extracellular surface (Figure 3) and are known as glycoproteins, a term that reflects the contribution of carbohydrate moieties to the protein structure.
Membrane Proteins
The relative size of different membrane proteins that contribute to cell membrane physiology and electrical excitability are shown in Figure 4. Some proteins such as G-protein linked receptors are not much larger than the thickness of the lipid bilayer, whereas others extend considerably into the intracellular or extracellular space. Typically, the smaller membrane proteins are composed of a single subunit, encoded by a single gene, whereas the larger proteins are an assembly of multiple subunits. This illustration shows each membrane protein in isolation, whereas in the cell the proteins are typically associated with other auxiliary proteins and are also tethered to the cytoskeleton.
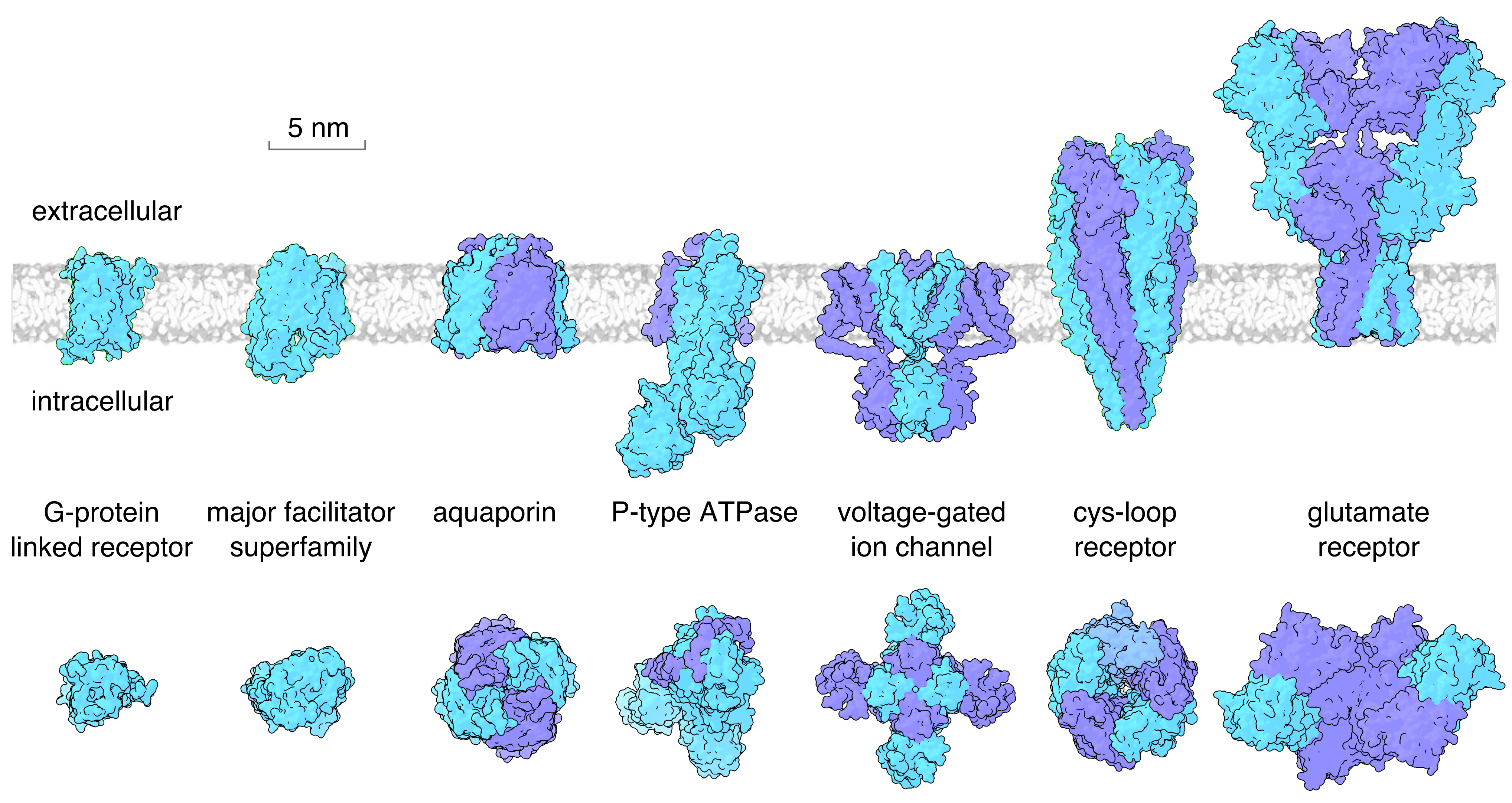
Figure 4 Examples of the main families of membrane proteins that contribute to cell membrane physiology and electrical excitability. Upper panel shows a side view with the lipid bilayer (extracellular surface to the top). The bottom panel shows a top view of the same proteins looking down from the extracellular side. Shown are a G-protein linked receptor (the M3 muscarinic receptor), a member of the major facilitator superfamily (the Glut1 glucose transporter), the AQP1 aquaporin, a P-type ATPase (the sodium-potassium pump), a voltage-gated ion channel (the Kv1.2 potassium channel), a cys-loop receptor (the nicotinic acetylcholine receptor), and the AMPA subtype ionotropic glutamate receptor.
Synthesis of Membrane Proteins
The synthesis of integral membrane proteins, particularly achieving the correct topology of the protein in the membrane, is a complex problem for the cell. Integral membrane proteins are synthesized by ribosomes that become tethered to a translocator complex (translocon) located in the endoplasmic reticulum (ER) membrane (Figure 5). Typically, the first transmembrane (TM) domain of the nascent peptide functions as a targeting sequence. This transmembrane sequence is recognized by the signal recognition particle (SRP), which in turn binds to the SRP receptor in the ER membrane. Following successful targeting to the ER membrane, the complex of ribosome and nascent peptide is transferred to the translocon. For proteins such as voltage-gated channels the amino terminus of the protein is located intracellularly and the first membrane spanning domain functions as a start-transfer signal, causing the translocon to mediate the translocation of the peptide trailing this transmembrane sequence through the translocon pore. This displaces a plug on the inner surface of the channel that normally gates the translocon channel. Transfer of the peptide continues until the translocon encounters a stop-transfer signal (typically the second membrane spanning domain) causing the translocon to stop transferring the peptide across the membrane, thereby allowing the peptide to accumulate on the cytoplasmic side. In general, alternating start and stop-transfer signals in the protein’s peptide sequence will combine to allow the channel to assemble with the correct membrane topology. This topology signaling can include cues from regions of the polypeptide outside the transmembrane domain of the protein and more complex schemes may be required to ensure that proteins with non-canonical transmembrane domains, such as voltage sensors and channel pores, can achieve the correct topology.
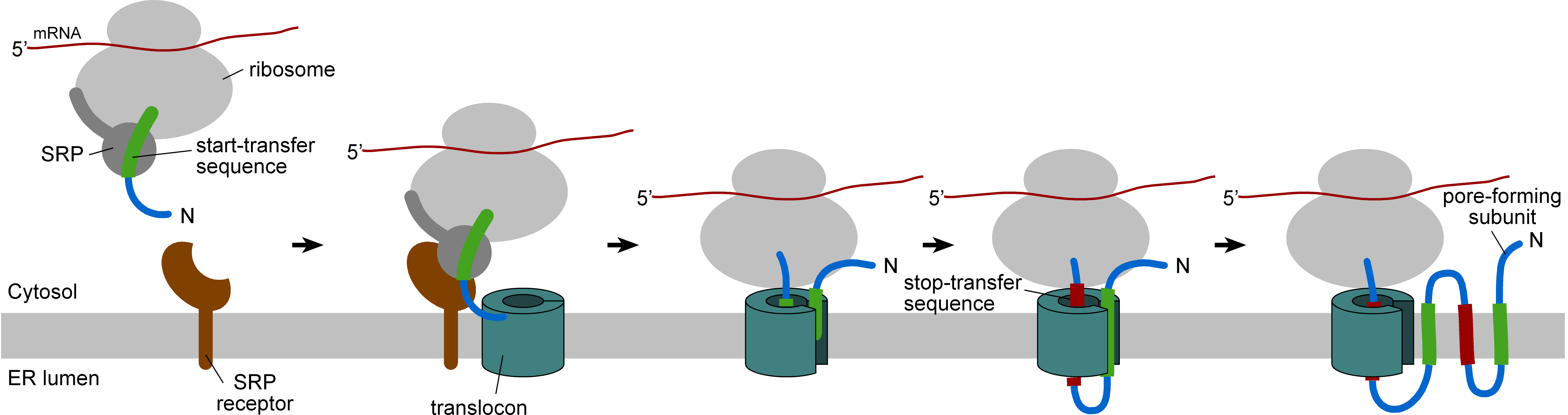
Figure 5 Most integral membrane proteins have hydrophobic transmembrane (TM) domains of 20–25 residues in length that form membrane spanning α-helices in the fully assembled protein (marked with red and green in the figure). The first of these TM domains acts as a targeting sequence to target the nascent peptide to the translocon in the ER membrane. This sequence is recognized by the signal recognition particle (SRP), which targets the entire complex to the ER membrane by binding to the SRP receptor. The ribosome and nascent peptide then attach directly to the translocon. The first TM domain is recognized as a start-transfer sequence by the translocon. This initiates movement of the downstream peptide through the translocon pore into the ER lumen. During subsequent translation of the protein the TM domains each signal to the translocon to start or stop transfer of the peptide across the ER membrane. The translocon has a lateral gate that can open to allow lateral transfer of each of the hydrophobic TM domains into the lipid bilayer.
In addition to having a trans-membrane pore for movement of the peptide across the membrane, the translocator complex is hinged and can open to allow the hydrophobic membrane spanning domains of the ion channel-forming protein to partition into the hydrophobic core of the membrane (Figure 5). By this means, the channel-forming protein is integrated into the lipid bilayer of the membrane.
Translocation of the growing peptide occurs co-translationally, meaning that the transfer of the protein into the ER lumen and membrane occurs at the same time as the protein is being synthesized. In general, the pore-forming subunit will undergo further maturation, aided by proteins associated with the translocon complex such as oligosaccharyl transferase as well as by ER resident membrane chaperones (such as calnexin). Glycosylation of the channel peptide by oligosaccharyl transferase also occurs cotranslationally and can contribute to establishing the correct topology.