Membrane Transport
If a solute can dissolve into the lipid bilayer (lipophilic) and in water, then it can cross the cell membrane by simple diffusion since the lipid bilayer does not present a significant barrier to diffusion (Figure 1). Oxygen, carbon dioxide, fatty acids and steroid hormones are all examples of nonpolar molecules that diffuse easily through the lipid domains of membranes.
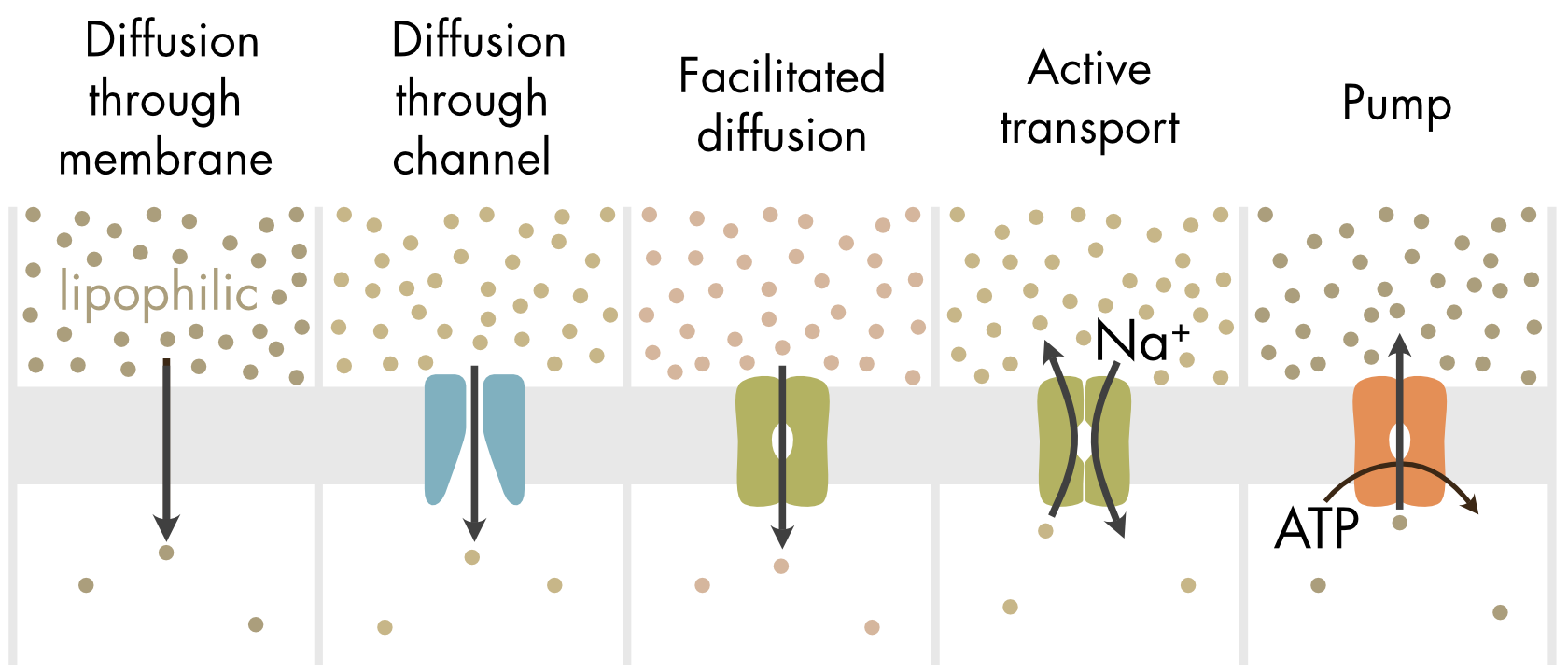
Figure 1 Mechanisms by which different solutes can move across the cell membrane.
The majority of molecules in biological solutions, however, cannot diffuse through the membrane or diffuse only poorly. For these polar or charged molecules the lipid bilayer represents an almost complete barrier to passive diffusion and a membrane protein intermediary is required in order for these solutes to cross the membrane. Cells have evolved multiple different transport mechanisms to move a diverse set of solutes across the cell membrane (Figure 1). These proteins fall into three major classes (Table 1).
Table 1 Classes of integral membrane proteins involved in transport of ions and polar molecules
| Protein | Function |
|---|---|
| Channels | facilitate diffusion of ions and water molecules down their concentration gradients by creating selective pores across the cell membrane |
| Transporters | two types, those that facilitate movement of solutes down their concentration gradient and those linked to ion gradients, which provide the energy to move solutes against their concentration gradients |
| Pumps | require energy in the form of ATP to move ions up their concentration gradients |
Many abundant charged ions can move across the cell membrane through specific ion channels down their electrochemical potential gradients. Similarly, water molecules can move passively through water channels. Facilitated diffusion requires specific transporters to move some solutes down their concentration gradient (Figure 1). Movement of solutes up a concentration gradient requires the expenditure of energy. Active transport by transporters uses energy stored in ion gradients, typically although not always gradients of sodium ions. Pumps use metabolic energy in the form of ATP to move solutes (typically ions) up a concentration gradient.
Common Properties of Membrane Transport Proteins
Channels, transporters and pumps share some common features. They are all integral membrane proteins with multiple membrane spanning alpha helical domains comprised of predominantly hydrophobic amino acids. They all effectively ‘wrap’ the solute in a protein sheath creating a pore across the lipid bilayer through which the solute can cross shielded from the membrane lipids. One or more specific solute binding sites are located in the pore. These are necessary to facilitate selective binding and passage of specific solutes and the exclusion of other solutes. In general mammalian channels, transporters and pumps share little structural similarity with each other. Exceptions to this include: some chloride channels that are closely related to Cl-/H+ exchange transporters, and the CFTR (cystic fibrosis transmembrane conductance regulator) chloride channel, which is a member of the ABC (ATP Binding Cassette) family of pumps.
A key difference between channels and transporters/pumps is the minimum number of gates across the pore that are required for normal function. Ion channels can function with a single gate (Figure 2). Once the gate opens, ions can flow rapidly through the open pore. In contrast, transporters and pumps require two gates to function correctly. Critically, only one gate can be open at any point in time or the solutes will start to flow back down their concentration gradient. Since cycling between the different states of the pump/transporter is relatively slow, solutes move through these proteins relatively slowly, with only one or a few molecules crossing the membrane with each cycle of gates opening and closing. As a consequence, channels move molecules across the membrane at much faster rates than transporters and pumps. When open, ions can move through ion channels at rates of 108 ions per second and water molecules can move through water channels as fast as 2x109 molecules per second. In contrast a typical transporter is much slower, moving solutes at rates of 200-50,000 molecules per second. Ion pumps are also relatively slow, due to the complex rearrangements of the pump protein during the pump cycle and typical rates are only a few hundred pump cycles per second.
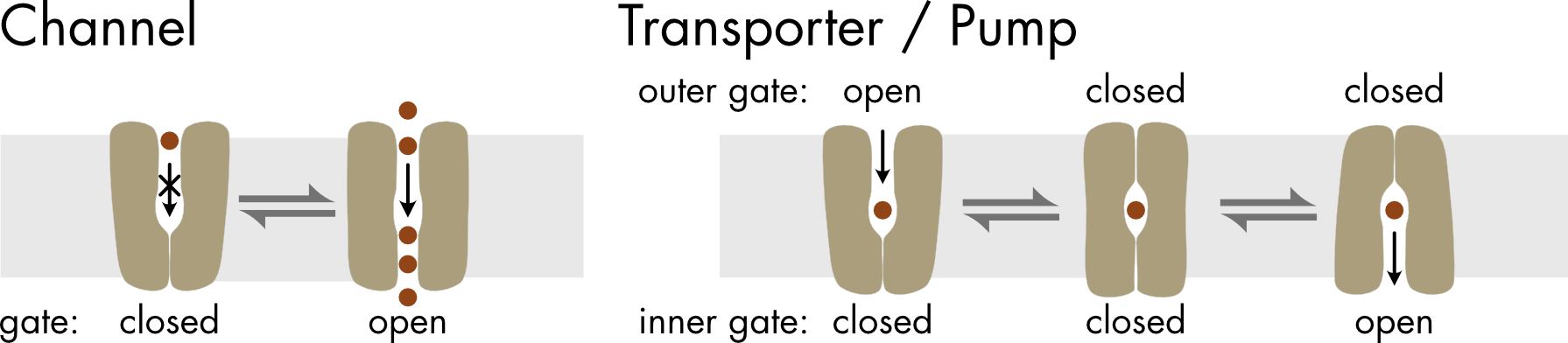
Figure 2 Comparison of the gating mechanisms for channels versus transporters and pumps.
Typically, most ion channels in the cell membrane will be closed most of the time whereas the transporters and pumps are generally constitutively active. This is necessary to maintain the balance between channel mediated ion fluxes down concentration gradients and pump/transporter mediated fluxes back up concentration gradients. Water channels are always open.
Distribution of Ions
The water in the body is divided into two main compartments: intracellular and extracellular. These two compartments are separated by the cell membranes of individual cells. Typical values for the concentration of the most common ions in these two compartments are given in Table 2.
Table 2 Ion concentrations in the intracellular and extracellular fluids of a typical mammal.
| Ion | Intracellular (mM) | Extracellular (mM) |
|---|---|---|
| Na+ | 15 | 140 |
| K+ | 130 | 4.5 |
| Ca2+ | 0.0001 | 1 |
| Mg2+ | 1 | 1 |
| Cl- | 7 | 116 |
| HCO3- | 15 | 26 |
| A- | 124 |
A- = fixed anions are the sum of all the negatively charged inorganic ions (sulfate and phosphates), proteins, and other organic molecules that are located inside the cell. Most of the Ca2+ and Mg2+ ions inside the cell are either sequestered within organelles or bound to proteins and it is the free ion concentration in the cytoplasm that is listed.
The specific values in Table 2 will vary among between different cells in the body and between different species, but the general principle is that all animal cells have a relatively high intracellular concentration of K+ ions, a low intracellular concentration of Na+ ions and a very low intracellular concentration of Ca2+ ions. The high concentration of NaCl in the extracellular solution reflects our origins as ocean living organisms. There is a high concentration of fixed anions inside the cell. These comprise the sum of all the compounds synthesized or sequestered by the cell, which have a net negative charge. These are called fixed because the cell membrane restrains them from diffusing out of the cell. These fixed solutes present an osmotic challenge for the cell and maintenance of the unequal distribution of ions between the inside and outside of the cell is essential to maintain osmotic balance and the integrity of the cell membrane. The ion gradients support the cellular physiology of every cell and the electrophysiology of electrically excitable cells.
Regulation of the ion and solute concentrations in the extracellular fluid requires the coordinated function of several major organ systems, including the kidney and cardiovascular system. Regulation of ion and solute concentrations within cells is the responsibility of each individual cell and a complex set of membrane transport proteins is found in the membrane of every cell (Figure 3).
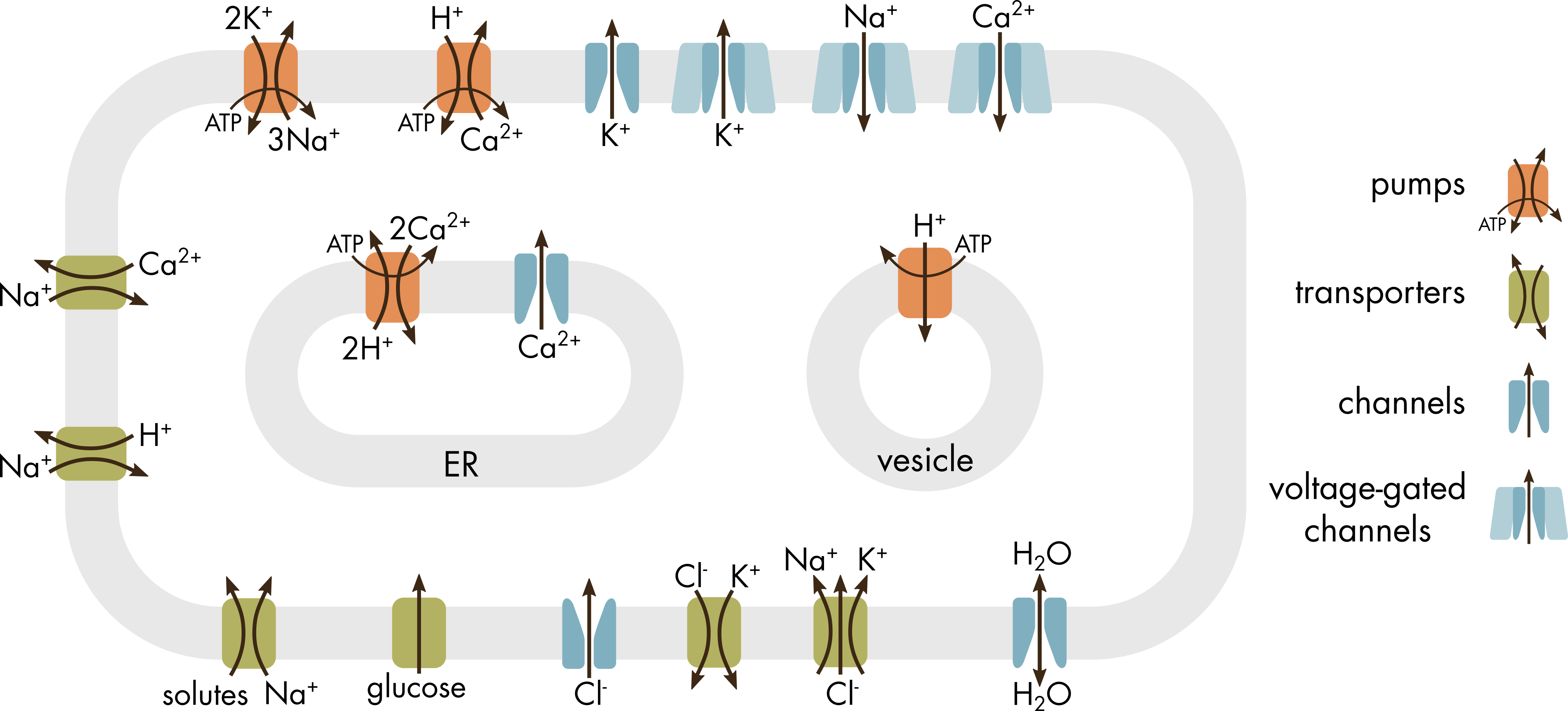
Figure 3 Membrane transport systems found in a typical electrically excitable cell. Transport of ions and solutes through the channels and transporters is reversible. The primary direction of transport in normal cells is shown by the arrows. Water is typically in equilibrium and flows in and out of the cell at similar rates.
Inspection of Figure 3 reveals that there are multiple apparently futile cycles—where an ion is pumped in or out of the cell with the expenditure of metabolic energy only for there to be multiple pathways for it to pass back across the membrane in the opposite direction. This is because in addition to maintaining osmotic balance ion gradients are used to provide energy for the transport of other solutes as well as for cell signaling, either electrical signaling via current flow through ion channels or chemical signaling via the influx of calcium ions into the cytoplasm.
The pump that moves sodium ions out of the cell and potassium ions into the cell, known as the Na,K-pump or Na,K-ATPase. This is arguably the single most important transport system in mammalian cells. It creates most of the nonequilibrium ion distributions, which produce electric potential energy, so important in electrically excitable cells, as well as the ion gradients that provide the chemical energy for secondary active transporters. In many cells it is the single biggest user of ATP.