Solutes
Water is a remarkably good solvent, into which a very diverse set of chemicals can dissolve. These molecules fall into three categories: ionic compounds (salts, acids and bases), polar covalent molecules, and nonpolar covalent molecules. Ionic compounds dissociate into ions in solution whereas the polar and nonpolar covalent molecules remain intact in solution. Ions and polar molecules are generally very insoluble in the lipid bilayer of the cell membrane. As a consequence, they do not easily cross cell membranes. Nonpolar molecules have variable solubility in lipid bilayers.
Ions
By itself pure water is a poor conductor of electricity. The conduction of electrical current by aqueous solutions is central to the electrical function of cells. Electrical conduction in biological solutions depends primarily on the number and nature of the charged ions found in that solution. These ions can dissolve in water because of the polar nature of water molecules. Water is generally a good solvent for ionic compounds, which include salts, acids and bases. These compounds all share the property of dissociating into ions when dissolved in water.
Ions in Solution
Two of the most common ions in the body are Na+ and Cl-. NaCl is common table salt. The need to regularly replenish these ions in the body is one reason why salty foods are perceived by animals as tasty. The NaCl molecule is uncharged and does not carry a current in its crystal form. In the NaCl crystal, the Na+ and Cl- ions are held together by ionic bonds between the positively charged Na+ ions and the negatively charged Cl- ions (Figure 1).
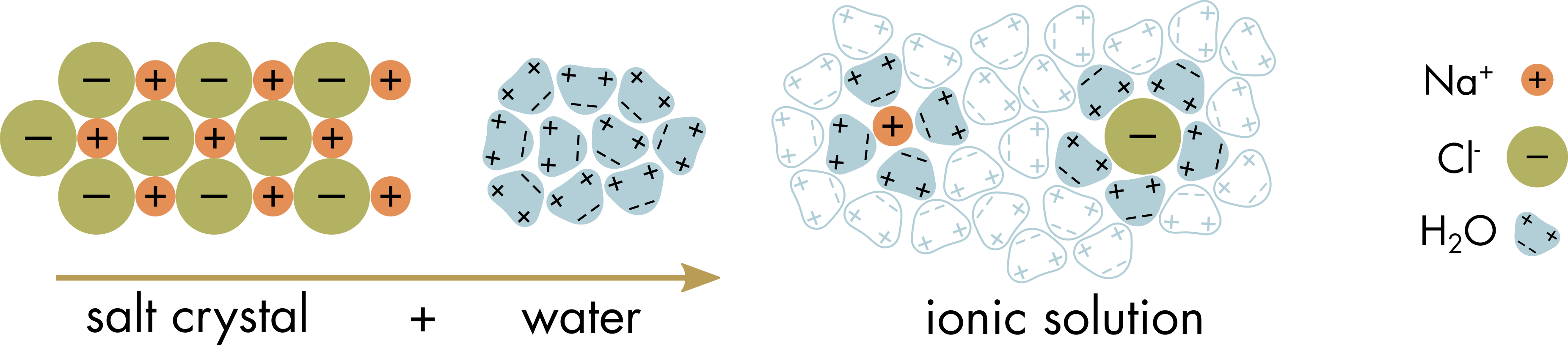
Figure 1 Dissociation of Na+ and Cl- ions in water. Some water molecules cluster tightly around the dissociated ions, drawn in by their relatively high charge, to create an inner hydration shell of oriented water molecules (indicated by the blue shaded water molecules). The orientation of the water molecules in the hydration shell is opposite for the cation and anion. Water molecules and the dissolved ions are in constant motion, so the hydration shell is a statistical concept, on average there are more oriented water molecules immediately adjacent to the ions.
Water can dissolve the NaCl crystal, just as it can dissolve most other ionic compounds (salts, acids, bases), because the dipolar water molecules can overcome the electrostatic interactions between the individual ions. The partial negative charge of the oxygen allows weak electrostatic binding with the positively charged Na+ ion and the partial positive charge on the hydrogen allows weak electrostatic binding with the negatively charged Cl- ion. The water molecules surround the ions, orienting themselves so that their positive poles face anions and their negative poles face cations. These transiently captured water molecules are known as hydration shells (Figure 1).
Dissociated ions are the primary current carriers in the body. They are known collectively as electrolytes, because of their ability to conduct electricity. Ions that have a net positive charge are called cations, while those that have a net negative charge are called anions. A failure of athletes to replenish electrolytes during endurance or other sporting events can produce disruption of the electrical activity in some of their electrically excitable cells, to potentially tragic effect if cardiac function is disrupted.