Water
The chemistry of water plays a central role in the biochemistry and physiology of all living beings. A striking thing about water is its ubiquity. Hydrogen and oxygen are the most and third most abundant elements in the universe respectively, and water appears to be the most abundant compound. During the period when life first evolved it is thought that earth was a water world, with a single ocean covering the entire planet. In human bodies 99% of the total number of molecules are water molecules, although they comprise only approximately 60% of the total body weight because water molecules are small relative to most biological molecules.
Water Chemistry
Given its centrality to life it is worthwhile reviewing the basic chemistry of water. Of the four pairs of valence electrons in the water molecule, two pairs are not involved in creating the bonds between the oxygen and hydrogen atoms (Figure 1). These two lone electron pairs repel the two hydrogen atoms creating a bond angle of approximately 105°. Unequal sharing of the other two pairs of valence electrons between the oxygen and hydrogen atoms results in a relatively large partial negative charge on the oxygen atom and a partial positive charge on each of the hydrogen atoms (Figure 1). The separation of these charges within the water molecule creates a dipole.
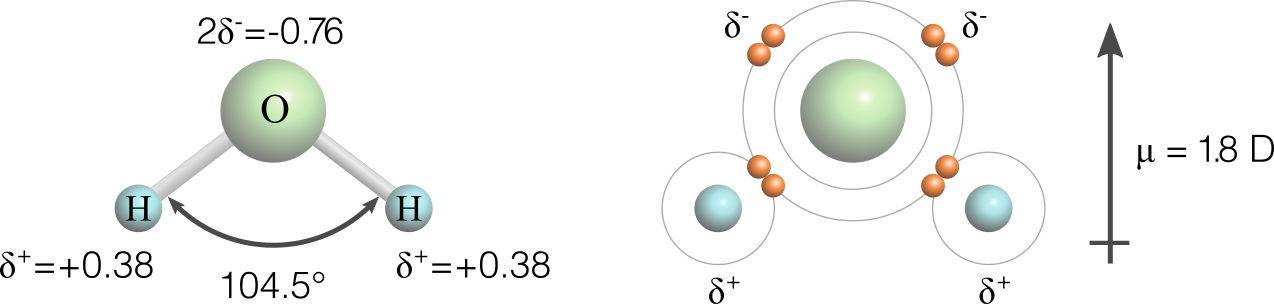
Figure 1 Water is a polar molecule with a partial negative charge (δ-) on the oxygen atom and partial positive charges (δ+) on the two hydrogen atoms. There are four electron pairs in the outer valence shell. Two of these are lone pairs and two are shared. The separation of the partial charges creates a molecular dipole (indicated by arrow) across the water molecule.
Water is a polar molecule, because of the uneven distribution of charge within the molecule. The bonds between the oxygen and two hydrogen atoms are polar covalent bonds. The nature of the bond formed by any two atoms depends upon the difference in the electronegativity of the atoms (Figure 2). For atoms with similar electronegativity values, such as hydrogen and carbon, there is close to equal sharing of the bonding electrons between the two atoms and there is no significant charge on either atom. Lipid molecules, which are composed primarily of these two elements, are nonpolar. In contrast, for ionic bonds there is complete transference of one or more valence electrons, creating ions that then bond by electrostatic attraction. Between these two extremes are polar covalent bonds, such as those found in water molecules. Atoms in polar covalent bonds share bonding electrons, like nonpolar covalent bonds, but share them unequally resulting in a significant partial charge on the atoms.
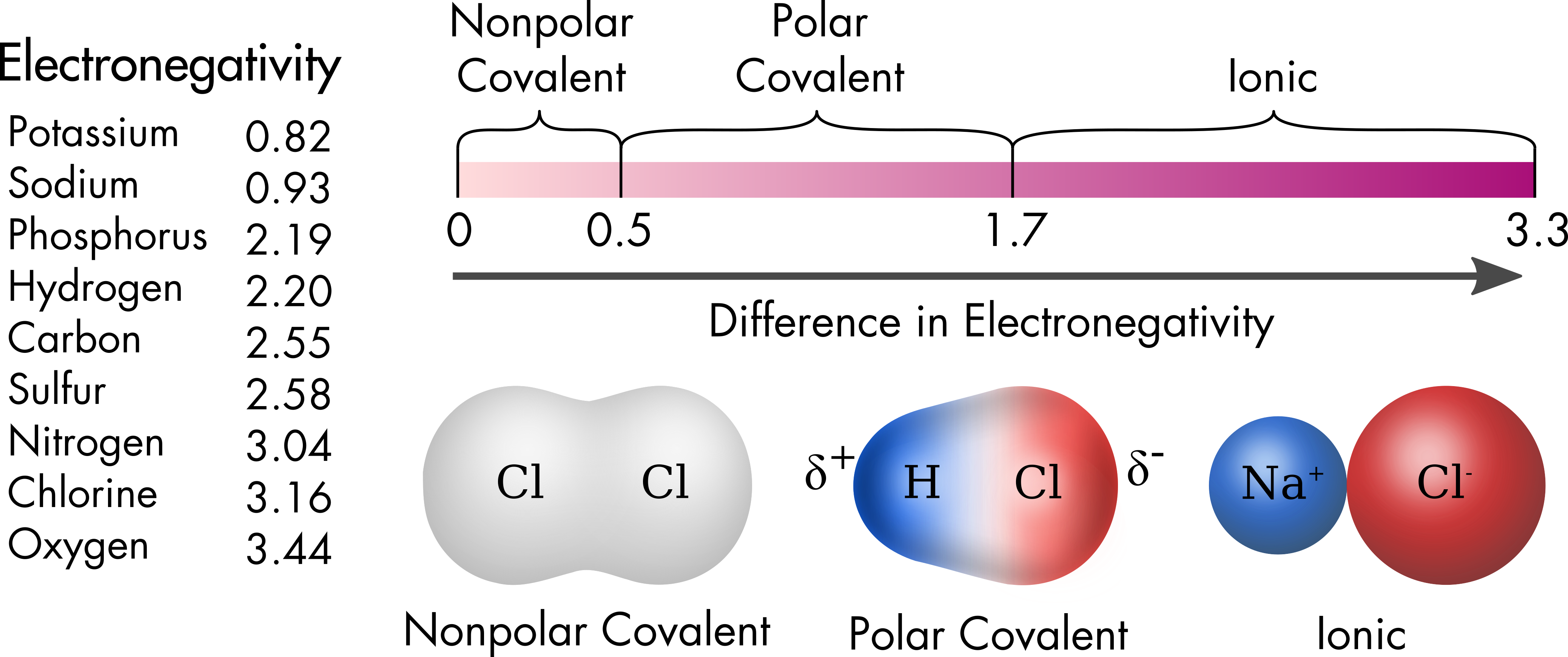
Figure 2 Electronegativity of common elements (Pauling scale). The type of bond that forms between two elements depends on the magnitude of the difference in electronegativity between the two elements. Differences in electronegativity less than 0.5 produce bonds that are mostly covalent with bonding electrons shared equally between the atoms. Differences larger than 1.7 such as between alkali metals (sodium or potassium) and halogens such as chlorine produce bonds that are mostly ionic. Polar covalent bonds have intermediate values and result in partial charges on the surface of the two atoms due to unequal sharing of the bonding electrons. Three compounds of chlorine are shown, each with a different partner and electronegativity difference. The red and blue surfaces of the molecules correspond to negatively or positively charged surfaces respectively. White corresponds to a neutral surface.
The effective shape of a molecule is generally defined by the distribution of electrons in the outer shell of the molecule since the spacing between molecules largely depends on repulsion between their outer electron clouds. An electron density isodensity surface is typically used to represent the surface of the water molecule, resulting in a blob-shaped molecule (Figure 3).
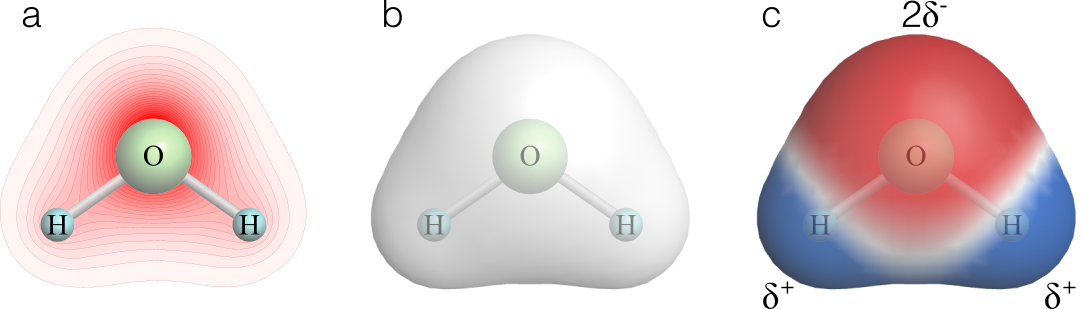
Figure 3 a) The greater electronegativity of the oxygen atom results in the electrons of the outer shell preferentially clustering around the oxygen atom. The average electron density around the oxygen atom (shown in red) is approximately 10x that around the hydrogen atoms. b) The surface of the water molecule is determined by how closely it can approach other molecules. This is largely determined by the electron density, since the electrons of each molecule will repel each other. An electron density isodensity contour (0.02 a.u., shown in gray) approximates the surface of the molecule. c) Electrostatic potential map. The distribution of charge in the molecule has been mapped onto the electron density contour. Red indicates electron-rich and blue indicates electron-poor regions.
The distribution of charge within the water molecule reflects the fact that the electrons cluster around the oxygen nucleus, leaving the hydrogen atoms partially denuded of electrons, and hence partially charged. When the electrostatic potential is mapped onto the surface of the water molecules, one side of the molecule has a positively charged surface and the other side has a negatively charged surface. The interactions between water molecules and between water molecules and solutes are largely determined by this property.
Hydrogen Bonds
Water molecules form hydrogen bonds between the partial positive charges on the hydrogen atoms and the partial negative charge on the oxygen atoms of adjacent molecules (Figure 4). The intermolecular hydrogen bond between an oxygen and hydrogen atoms of adjacent water molecules is approximately one twentieth the strength of the intramolecular covalent bond between the water molecule’s oxygen and hydrogen atoms. There are, however, a lot of hydrogen bonds linking water molecules together, and their presence underlies many of the chemical properties of water.
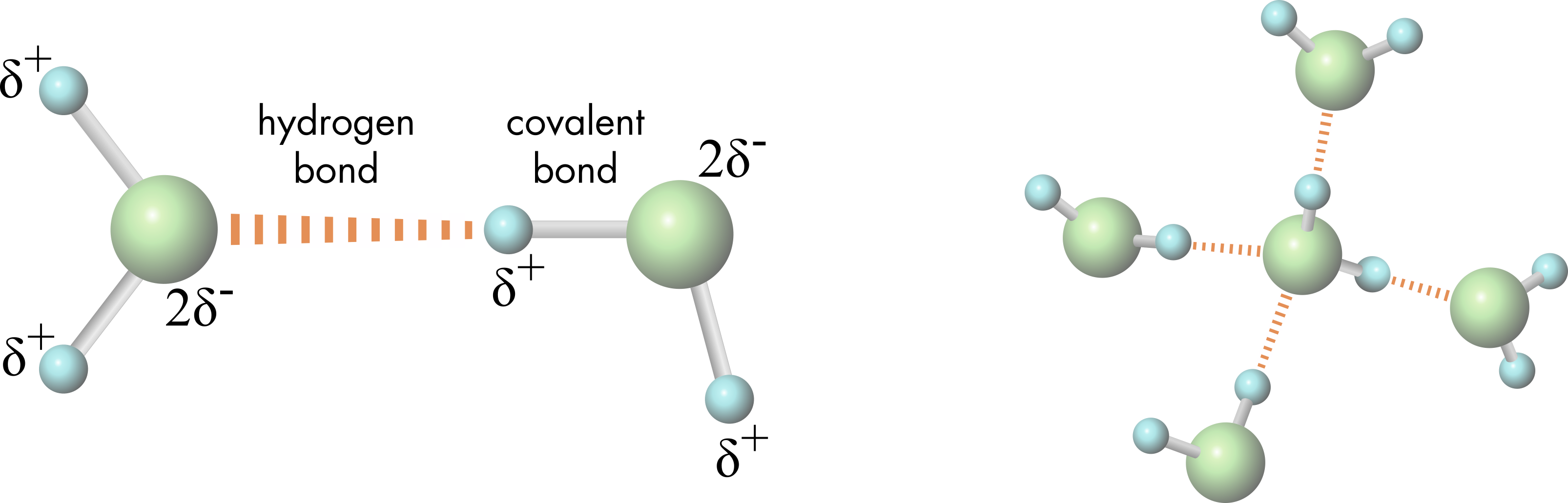
Figure 4 Hydrogen bonding between two water molecules (left panel). Tetrahedral arrangement of a water molecule and four hydrogen bonded partners (right panel).
Since the angle between the two covalent bonds of water is about 105°, groups of hydrogen-bonded water molecules form tetrahedral arrangements (Figure 4), although the water molecules are in constant motion, so any structure is very transient. The local structuring of water molecules created by hydrogen bonding accounts for some unusual phenomena such as the self-assembly of lipid bilayers in aqueous environments. The water molecules’ strong preference for interacting with each other results in the exclusion of hydrophobic molecules from the aqueous phase, favoring an arrangement where hydrophobic molecules cluster together to minimize disruption of hydrogen bonding between water molecules.
Deprotonation of Water
Water molecules are somewhat unstable, losing or gaining a proton on average every millisecond. Protons are not stable in aqueous solutions and almost instantly react with a surrounding water molecule. The most likely outcome of a deprotonation event is the formation of a hydronium ion (H3O+) and a hydroxide ion (OH-).
\[\text{H}_{\text{2}}\text{O\ +\ }\text{H}_{\text{2}}\text{O\ \ }\text{⇌}\text{\ \ H}_{\text{3}}\text{O}^{\text{+}}\text{\ +\ O}\text{H}^{\text{-}}\text{\ }\]
A consequence of this deprotonation is that there is a low resting concentration of hydronium and hydroxide ions in nominally pure water. These charged molecules account, in part, for the low but non-zero electrical conductivity of pure water. In biology textbooks hydronium ions and related species (H3O+(H2O)n)) are referred to collectively as hydrogen ions, a convention that we will also use.
The other factor contributing to the electrical conductivity of water is proton-hopping. As the name implies, protons can jump from one water molecule to another, contributing to current flow and the movement of protons through pure water. The water channels, known as aquaporins, found in cell membranes appear to have evolved to minimize this phenomenon, which would otherwise create an unregulated pathway for movement of protons through these channels and across the membrane.