Evolution of Cells
When you look at a cell, even one newly formed, you are, in one sense, looking at something unimaginably ancient. Like a tale from Norse mythology that has been repeated for a thousand years by a constantly changing cast of Viking poets, the cell is a story that has been told and retold, not just for millennia but for billions of years. Even with the passage of so much time, however, the overall arc of the story has remained surprisingly constant, and critical elements of the story have remained almost unchanged throughout that time.
When researchers first began sequencing prokaryote genomes one unexpected finding was that many of the proteins as well as much of the cellular organization that underlies the sophisticated electrical signaling functions of neurons was evolutionarily very ancient. In many cases considerable sequence and functional similarity can be found between the proteins used by human brains for electrical signaling and proteins found in prokaryotes. This is due in large part to the fact that cells, of all kinds, have had to solve a common set of cell physiological problems ever since the evolution of the first cells. These problems include capture and storage of energy, transport of solutes into and out of the cell, maintenance of ion balance, maintenance of osmotic balance and sensing of the environment. The proteins that underlie these functions have been elaborated and modified during the course of eukaryote evolution, but in large part it is changes in the organization of cells and the development of multicellularity rather than the evolution of completely novel proteins that accounts for the complex electrical function found in the neurons and muscle cells of animals.
In this section we address how the first cells may have evolved and the various constraints on this process. It should be noted that the events leading to the evolution of cellular life are very poorly understood. This ancient, apparently unique, historical event cannot be readily replicated, making it inaccessible for systematic study. Consequently, most writing on this topic remains speculative.
Relationships between the Domains of Life
Before considering the evolution of cell and membrane physiology it is useful to understand, in broad outline, how the three domains of life, bacteria, archaea and eukaryotes, are related (Figure 1). The common ancestor to bacteria and archaea is known as the last universal common ancestor (LUCA). The precursors to LUCA are known as protocells.
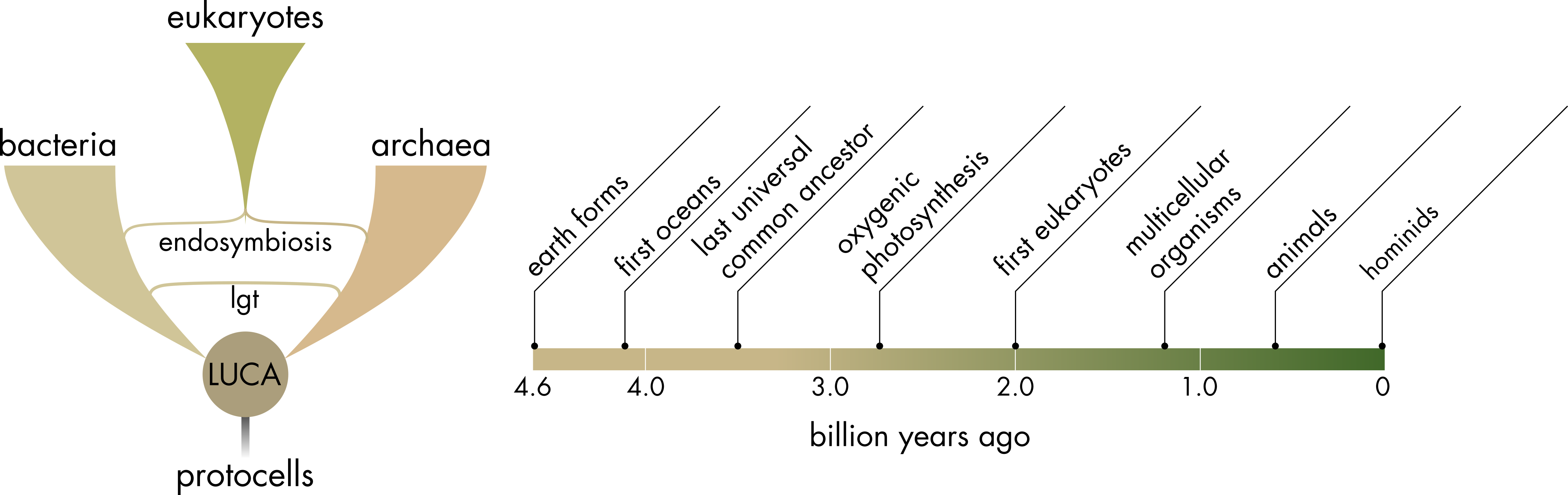
Figure 1 Evolutionary relationship between the three domains of life. The last universal common ancestor (LUCA) is shown at the base of the split between the bacterial and archaeal domains. Lateral gene transfer (lgt), both within the bacterial and archaeal lineages and between the two lineages, had a significant effect on evolutionary history, sharing useful genetic innovations laterally between prokaryotic lineages. This process was invaluable in allowing prokaryotes to adapt to a wide array of different environments, it does however greatly complicate the determination of phylogenetic relationships amongst prokaryotes. Timeline shows key events. When cellular life first appeared is uncertain but seems to have occurred relatively soon after the formation of the first oceans. The last common ancestor of bacteria and archaea also appeared relatively quickly but there appears to have been a long delay before the appearance of the first eukaryotes.
Based on shared properties between bacteria and archaea, the last universal common ancestor appears to have had relatively sophisticated cell and membrane physiology associated with energy capture. Given the apparent difficulty of the task, LUCA evolved surprisingly quickly. There is a relatively narrow window from the formation of the first oceans (approximately 4.2 billion years ago, although possibly slightly earlier) and the appearance of LUCA, approximately 3.5 billion years ago (Figure 1). Adding to the degree of difficulty, for much of this period earth was subject to a significant meteorite bombardment, which was much more common during this period than in later stages of earth’s existence. These bombardments may have been sufficiently intense to completely sterilize earth on one or more occasions.
Eukaryotes evolved from an archaeal species that was host to endosymbiotic bacteria. These endosymbiotic bacteria eventually evolved into the mitochondria found in almost all extant eukaryotic cells. The merger of the archaeal and bacterial lineages that lead to the first eukaryotes was a very complex process, it was not simply an archaeal host taking in a bacterial cell as an organelle. Eukaryotic cells developed a vastly more complex cellular organization than had been previously found in either bacteria or archaea. The genome of the stem eukaryote contained a mixture of bacterial and archaeal genes, with bacterial genes being predominant, in addition to many newly evolved genes associated with eukaryote specific innovations. These innovations include the nuclear membrane, introns and splicing, internal membrane systems, the cytoskeleton and sexual reproduction. Eukaryotic cells remain chimeras in that they possess both archaeal ribosomes in the cytosol and bacterial ribosomes in mitochondria.
Evolution of the First Cells
It remains uncertain how life started from rocks, H2, CO2 and water. A distinction is usually made between the abiotic organic chemical reactions that are presumed to have proceded the evolution of the first living organisms and life itself. Arguably the dividing line was the appearance of structures with an impermeable and continuous cell membrane. Before the existence of membrane delimited structures, it would have been difficult, or impossible, to define discrete individuals. The cell membrane establishes the basic delineation between self and non-self, creating the potential for Darwinian competition between individuals. From the time when the first protocells appeared it is reasonable to believe that natural selection can explain all the subsequent steps to more complex and diverse life forms.
The cell membrane’s critical role in the evolution of life comes about because it performs functions that are somewhat analogous to the property and patent laws of a capitalist economy. Like the property laws, the cell membrane distinguishes private property, what is inside the cell, from common property, everything outside the cell. This allows the cell to concentrate useful resources inside the cell (e.g. ATP, glucose) restricting them for the private use of the cell.
An equally important property is that the cell membrane allows the cell to effectively patent any novel innovations occurring in that cell’s genetic material by restricting the sharing of novel proteins and metabolic products. If a cell has an advantageous mutation in its genetic material, that cell and its progeny will retain sole rights to the benefits afforded by that mutation for some time, potentially conferring a competitive advantage to cells in this lineage relative to other cells.
Gibbs Free Energy and Energy Fluxes
However life started there is one certainty, it required a steady flux of energy from the environment in order to counteract the second law of thermodynamics, which states that entropy only increases in a closed system. The second law causes cells to inevitably break down over time and a continuous flux of energy is necessary to allow the cell to maintain and repair itself, to grow, and to reproduce. Cells are by necessity open systems taking both energy and material from their environment.
Arguably, the property that is most central to understanding how life works is Gibbs free energy,
\[\Delta G = \Delta H - T\Delta S\]
where, \(\Delta G\) is the change in Gibbs free energy, \(\Delta H\) is the change in enthalpy, a measure of heat given off (or taken up) by a given process, \(T\) is the temperature and \(\Delta S\) is the change in entropy. The entropy (\(\Delta S\)) is negative if the system becomes more ordered and is positive if the system becomes more disordered.
For a biological process, or any natural process, to proceed spontaneously the change in free energy, \(\Delta G\), must be negative. This means that the process must either produce an increase in entropy or there must be an expenditure of energy to produce heat, \(\Delta H\), that exceeds any decrease in local entropy. The heat is released from the biological system into the environment, producing a net increase in entropy.
Some biologically important processes do result in an increase in entropy. Diffusion and the self-assembly of proteins and lipid bilayers are examples of processes that will go forward in the absence of an input of energy. However, the net effect of all the biochemical and physiological processes in the cell is a decrease in entropy within the cell. Thus, life requires a continuous expenditure of energy in order to maintain a negative \(\Delta G\). This energy is derived in one form or another from the environment. The first cells appear to have harnessed geochemical energy through the oxidation of reduced compounds (hydrogen, hydrogen sulfide, methane) produced by water-rock reactions. Later, reduced carbon compounds from dead cells and cellular waste products would also have been available for oxidation. Later again, although exactly when is uncertain, cells began to utilize sunlight as an energy source through the evolution of anoxygenic photosynthetic reactions. Oxygenic photosynthesis, which produces oxygen, evolved later again resulting in the gradual buildup of oxygen concentrations in the atmosphere and ocean. Oxygen, when utilized as an electron sink, releases considerably more energy per electron transfer than any other commonly available electron sink. The appearance of oxygen and its subsequent use as an electron sink made respiration much more productive than it had been previously and thereby greatly increased the amount of energy available to the biosphere.
Energy Fluxes and Ion Fluxes
There appears to have been a very long evolutionary history pairing cellular energy fluxes and the fluxes of ions across cell membranes. Cells can conserve energy from the environment in one of two ways, either by respiration, in which the generation of ion gradients is a crucial step in the formation of ATP, or by fermentation, in which ATP is formed solely by enzymatic pathways. Respiration is ubiquitous in cells because it is considerably more efficient than fermentation, which is used as the primary energy generating pathway by only a very few organisms. Respiration can be anaerobic (oxygen independent) or aerobic (oxygen dependent). Evolutionarily, anaerobic respiration predates aerobic respiration, if for no other reason than that there was little or no free oxygen in the atmosphere and oceans prior to the evolution of oxygenic photosynthesis (Figure 1). Aerobic respiration evolved first in prokaryotes including the precursors of eukaryotes. Eukaryotes almost universally rely on aerobic respiration to fulfil their energy demands.
The same basic mechanism, utilization of the chemical potential energy stored in an ion gradient, underlies both anaerobic and aerobic respiration (Figure 2). Typically, a gradient of hydrogen ions is generated across an ion impermeant membrane, although sodium ion gradients can be used by some prokaryotes. The chemical potential energy stored in this gradient is then exploited by the enzyme ATP synthase to generate ATP. The movement of ions down a chemical gradient to generate ATP is known as chemiosmosis. The enzyme ATP synthase is an unusually complex molecular machine. Its complexity is regularly used as an argument for the existence of God. The structure of ATP synthase is conserved across all domains of life and appears to have been present in LUCA, approximately 3.5 billion years ago.
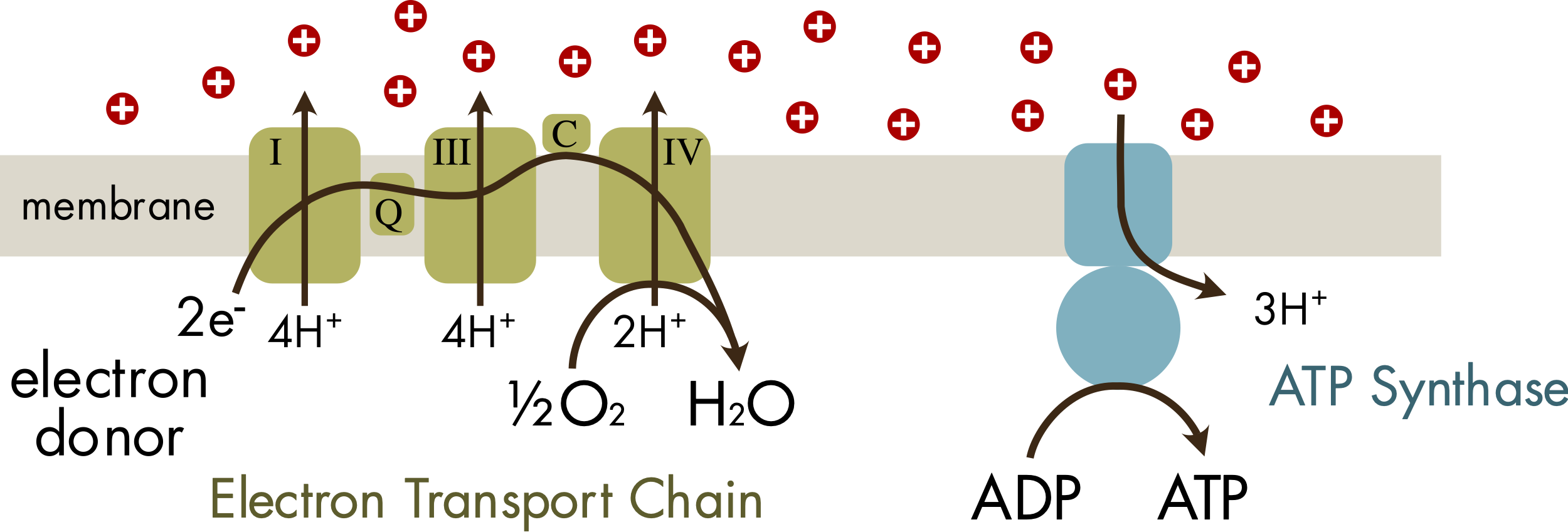
Figure 2 Respiration with an electron transport chain, hydrogen ion gradient (red spheres) and ATP synthase as found in the mitochondria of eukaryotes. There are three main protein complexes in the chain, complexes I, III and IV. Two smaller proteins ubiquinone (Q) and cytochrome c (C) transfer electrons between the main complexes. For each pair of electrons that passes through the chain 10 hydrogen ions are pumped across the membrane to create a gradient in hydrogen ions. Anaerobic respiration uses a similar mechanism although the electron acceptor is not oxygen and less energy in the form of pumped hydrogen ions is harvested by each electron transfer. Three hydrogen ions are transferred back across the membrane by ATP synthase for each ATP molecule created from ADP and inorganic phosphate.
In all eukaryotes and most extant prokaryotes the gradient in hydrogen ions is generated by the electron transport chain (Figure 2). This catalyzes a redox reaction with an electron donor, or source, and an electron sink. In mitochondria the most common electron donor is NADH, which is derived from digested food, and the electron sink is oxygen, which is converted to H2O. In mitochondria the electron donor molecule donates electrons to the first of three main protein complexes that comprise the electron transport chain. Electrons are transferred down this chain via a series of redox reactions until they are finally donated to the electron sink. This electron transfer is tightly coupled to the transport of protons across the membrane, which creates the gradient of hydrogen ions across the membrane.
Respiration is considerably more complex in prokaryotes although the same basic chemiosmotic system is used. Many prokaryotes can perform both aerobic and anaerobic respiration and may use multiple different electron transport chains in the membrane to exploit a variety of different energy sources. An important difference between prokaryotes and eukaryotes is the spatial organization of the system. In prokaryotes the proton gradient is generated across the plasma membrane whereas in eukaryotes the proton gradient is generated across the convoluted inner mitochondrial membrane. The pumped protons remain sequestered within the mitochondria by the outer membrane of the mitochondria.
It is uncertain how LUCA generated the ion gradients used by its ATP synthase. Current research suggests that it may have been a methanogen, utilizing carbon dioxide as a terminal electron acceptor in an electron transfer chain, although this remains speculative. The near universality of chemiosmosis in the harvesting of energy fluxes appears to have several causes, i) it is an efficient mechanism for capturing the energy released by redox reactions, ii) it is very flexible in both how ion gradients can be generated and the energy sources (redox pairs) that can be used to generate the gradients, iii) it can summate energy from redox reactions that by themselves produce insufficient energy to generate an ATP molecule directly. An example of the flexibility and modularity of this mechanism is that chemiosmosis is also used in photosynthesis to generate ATP.
Consequences of Using Chemiosmosis for Energy Capture
For chemiosmosis to be reasonably efficient the cell membrane must be ion impermeant, at least to sodium ions, but in most cases also to hydrogen ions. As a consequence, early cells most likely had a lipid bilayer membrane that could act as an electrical insulator i.e., could block the movement of current carrying ions. It is relatively difficult to make lipid bilayers sufficiently impermeant that they block hydrogen ions from crossing and this constraint will necessarily result in a very low permeability to most charged and polar solutes. Therefore, it appears likely that multiple specific solute transport systems must have also evolved early to move solutes in and out of the cell. There is evidence that LUCA had a Na+/H+ antiporter, which presumably acted to stabilize the concentrations of these cations within the cell.2
Due to the pumping of hydrogen ions mitochondria have a large electrical potential across the inner membrane, of the order of -150 mV, and prokaryotes have a similar voltage across their cell membranes. A membrane potential of this size creates a very large electric field across the thin lipid bilayer that would have also created specific constraints on membrane structure. For extant cells if the membrane potential exceeds approximately 200 mV in magnitude the electric field produces dielectric breakdown of the membrane creating holes in the membrane. It is likely that the problem of membrane breakdown in high electric fields would have favored the early evolution of ion channels in the membrane to stabilize the membrane potential in the face of variable rates of ion pumping, which depend on the availability of energy sources. These channels would most likely have been selective against protons and/or sodium ions to avoid degrading the chemiosmotic ion gradients, which may have favored the early selection of potassium channels.
Because of these various constraints it appears that the earliest cells had to evolve a package of features to support energy capture (Table 1). The pathway to the acquisition of this complex set of features in the first cells is not well understood.
Table 1 Chemiosmosis ‘Toolkit’
| Ion impermeant lipid bilayer which acts as an electrical insulator |
| Ion pumps to create ion gradients |
| Transporters to move solutes across the impermeant membrane |
| Potassium ion selective channels to stabilize membrane potential |
| ATP synthase |
Another other membrane physiology related property that is universal is a high internal potassium ion concentration. Exactly why this property evolved is also uncertain. Early evolution of potassium selective channels for membrane potential stabilization combined with the negative membrane potential produced by ion pumping would automatically result in a high internal concentration of potassium ions.3 Extant enzyme systems and mechanisms for packing of nucleic acids depend on high intracellular potassium ion concentrations for their function. It is possible that once a high internal potassium concentration became established in early cells most biochemical systems evolved to become dependent on high potassium concentrations. This would have made subsequent selection of any other pattern of ion distribution unlikely.
It is fortunate for all of us that these membrane physiological properties that initially evolved to facilitate energy capture are compatible with the evolution of electrical excitability and in fact facilitate the evolution of complex electrical signaling.
References
- Judson, O. P. (2017) The energy expansions of evolution. Nat Ecol Evol 1, 138.
- Weiss, M. C. et al. (2016) The physiology and habitat of the last universal common ancestor. Nat Microbiol 1, 16116.
- Korolev, N. (2020) How potassium came to be the dominant biological cation: of metabolism, chemiosmosis, and cation selectivity since the beginnings of life. BioEssays 10.1002/bies.202000108
Further Reading
Lane N. The vital question: energy, evolution, and the origins of complex life, 2016.