Synaptic Transmission in the Central Nervous System
The number of synapses on a neuron ranges between 100 and 100,000 with a typical value being 10,000. For a given connection between two cells, however, the number of synaptic connections is relatively low, of the order of 1-25 synaptic contacts (Figure 1).
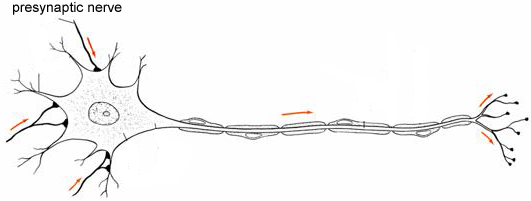
Figure 1 Neuron showing three different presynaptic nerves synapsing on its dendritic tree. For clarity, most synaptic connections are not shown.
The connections between a given presynaptic nerve and the postsynaptic cell in the CNS are generally quite small; epsps are only of the order of 1 mV in size, much smaller than seen at the NMJ. As a consequence, when a single pre-synaptic axon fires the post-synaptic cell will generally not fire. Synaptic transmission in the CNS generally requires the coordinated actions of a number of cells acting on the post-synaptic neuron rather than the relatively simple 1:1 mapping between pre- and post-synaptic firing that occurs at the NMJ. This co-ordination is known as summation. There can be both temporal summation and spatial summation between synaptic inputs to bring the membrane potential to threshold (Figure 2).
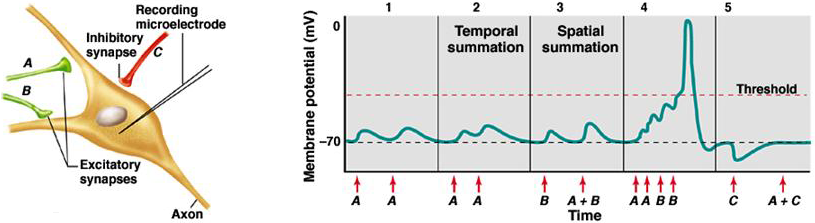
Figure 2 Synaptic transmission in the CNS.
Temporal summation means summation over time. Repetitive firing of the same synapse causes the membrane potential to become more depolarized than if the synapse fired more slowly. This phenomenon is due in part to the membrane capacitance, which slows down the voltage response to a synaptic current, spreading the response over time and allowing summation with subsequent epscs. Spatial summation simply means the summation of more than one synaptic input firing simultaneously.
Excitatory Synapses in the CNS
The receptors at most excitatory receptors in the CNS are glutamate receptors. There are two types of glutamate receptors that are of particular importance: the AMPA receptors and the NMDA receptors (Table 2).
Table 2 Glutamate Receptor Genes
| AMPA Receptors | |
|---|---|
| Genes | GluA1 GluA2 GluA3 GluA4 |
| Properties | main form of GluR |
| fast kinetics | |
| fast opening, closing and desensitization | |
| most native receptors in principal neurons contain the GluA2 subunit | |
| receptors containing the GluA2 subunit have low Ca2+ permeability and small conductance |
| NMDA Receptors | |
|---|---|
| Genes | NR-1 NR-2A NR-2B NR- 2C NR-2D |
| Properties | all receptors contain the NR-1 subunit, which has the agonist binding site |
| slow kinetics | |
| large single channel conductance | |
| high Ca2+ permeability | |
| channel blocked by Mg2+ ions |
Pharmacology of Glutamate Receptors
Initial progress in understanding the function of excitatory synapses in the CNS depended on the development of specific synthetic agonists and antagonists to the glutamate receptor. There are two main types of glutamate receptors the AMPA receptor and NMDA receptors. The AMPA receptors are activated by a specific agonist known as AMPA (hence the name) and blocked by the specific antagonist CNQX (Table 3). The NMDA receptors are activated by NMDA, which does not activate the AMPA receptors. NMDA receptors can be pharmacologically blocked with APV.
Table 3 Glutamate Receptor Pharmacology
| Agonist | Antagonist | |
|---|---|---|
| AMPA Receptors | AMPA | CNQX |
| NMDA Receptors | NMDA | APV |
AMPA Receptors
The AMPA receptors function similarly to the AChRs of the NMJ. They are cation channels with relatively fast kinetics. They are responsible for mediating most of the synaptic current at glutaminergic synapses at membrane potentials below threshold.
In principal neurons most AMPA receptors contain the GluA2 subunit. These receptors are impermeable to calcium ions and have a linear current-voltage curve with a reversal potential close to 0 mV. These properties are dependent on RNA editing of the GluA2 subunit, conversion of a glutamine to an arginine residue in the pore loop, which is known as Q/R editing. In contrast, in local circuit neurons, a large fraction of the receptors lacks the GluA2 subunit. This form of the AMPA receptor is calcium permeable, have a non-linear I-V relationship and higher single-channel conductances.
NMDA Receptors
The NMDA receptors are permeable to Ca2+ ions. Calcium flux through the NMDA receptors is very important in modulating the strength of synaptic connections.
The function of the NMDA receptor is considerably more complicated than the AMPA receptor. A key property is that the receptor is gated by both agonist and voltage (via Mg2+ block). The current-voltage curve for the NMDA receptor is non-linear. In the depolarized region, at normal Mg2+ concentrations (0.5 mM), the current-voltage curve has a negative slope (Figure 3). At positive potentials it is close to linear. The reason for this is that external Mg2+ ions block the channel by binding in the pore of the channel. If the external Mg2+ ions are removed the current-voltage curve becomes linear over most of the voltage range.
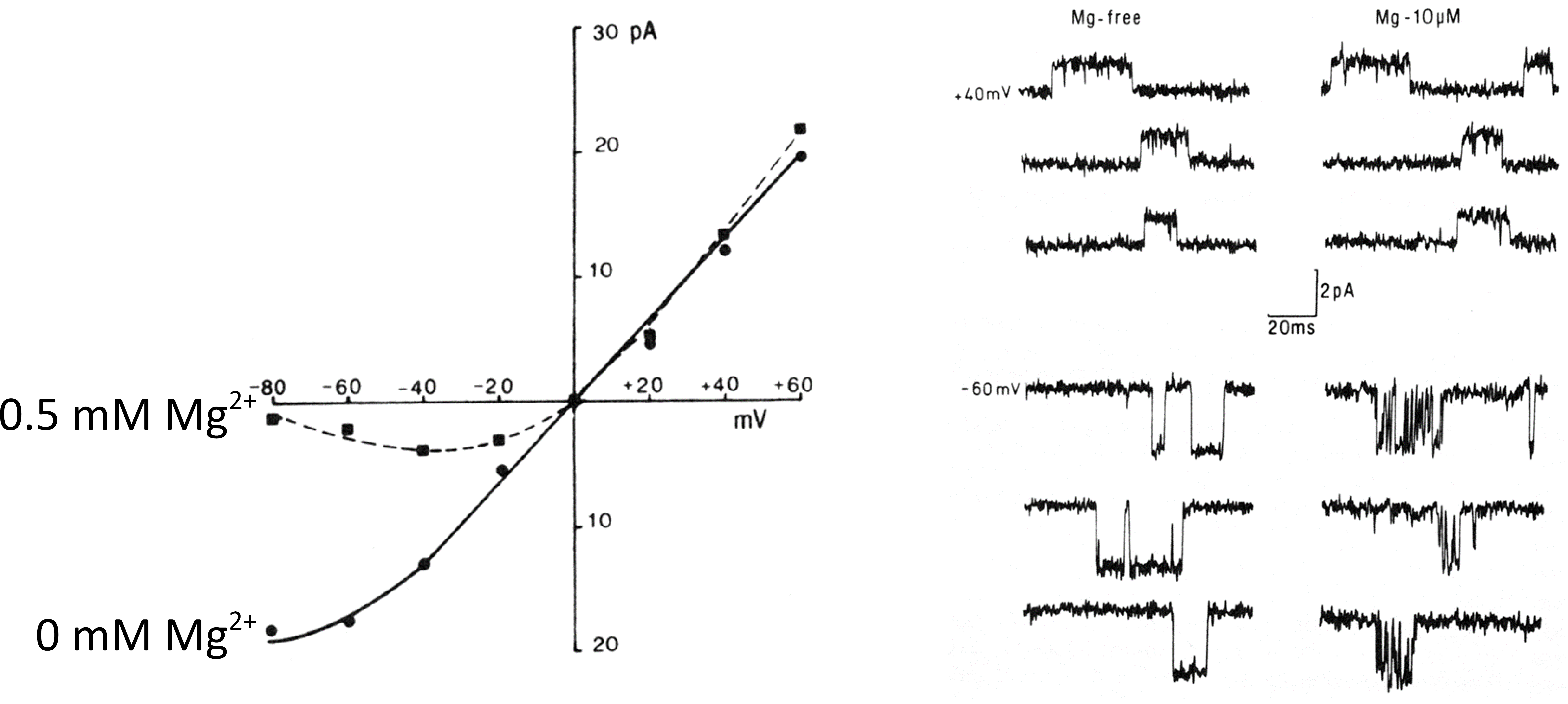
Figure 3 Current-voltage curve for the NMDA receptor in the presence and absence of external Mg2+ (left panel). Mg2+ block of single channel currents (right panel).
At the single channel level, in the absence of Mg2+ ions the channel openings are relatively simple (Figure 3). In the presence of Mg2+ ions however the channels openings are very short and spiky and occur in bursts, which is characteristic of channel blocking by ions.
Synaptic Transmission at Glutaminergic Synapses
AMPA and NMDA receptors are both found at the same synapse for many excitatory synapses. At synapses where both are present, when glutamate is released from the presynaptic terminal both classes of receptors can be activated simultaneously.
Under normal conditions, at negative membrane potentials, it is primarily the AMPA receptors that open and contribute significantly to the synaptic current because the NMDA receptors are largely blocked by Mg2+. At positive potentials the NMDA receptors are no longer blocked and they make a much larger contribution to the synaptic current resulting in much slower decay of the synaptic current, reflecting the slower kinetics of the NMDA receptor (Figure 4).
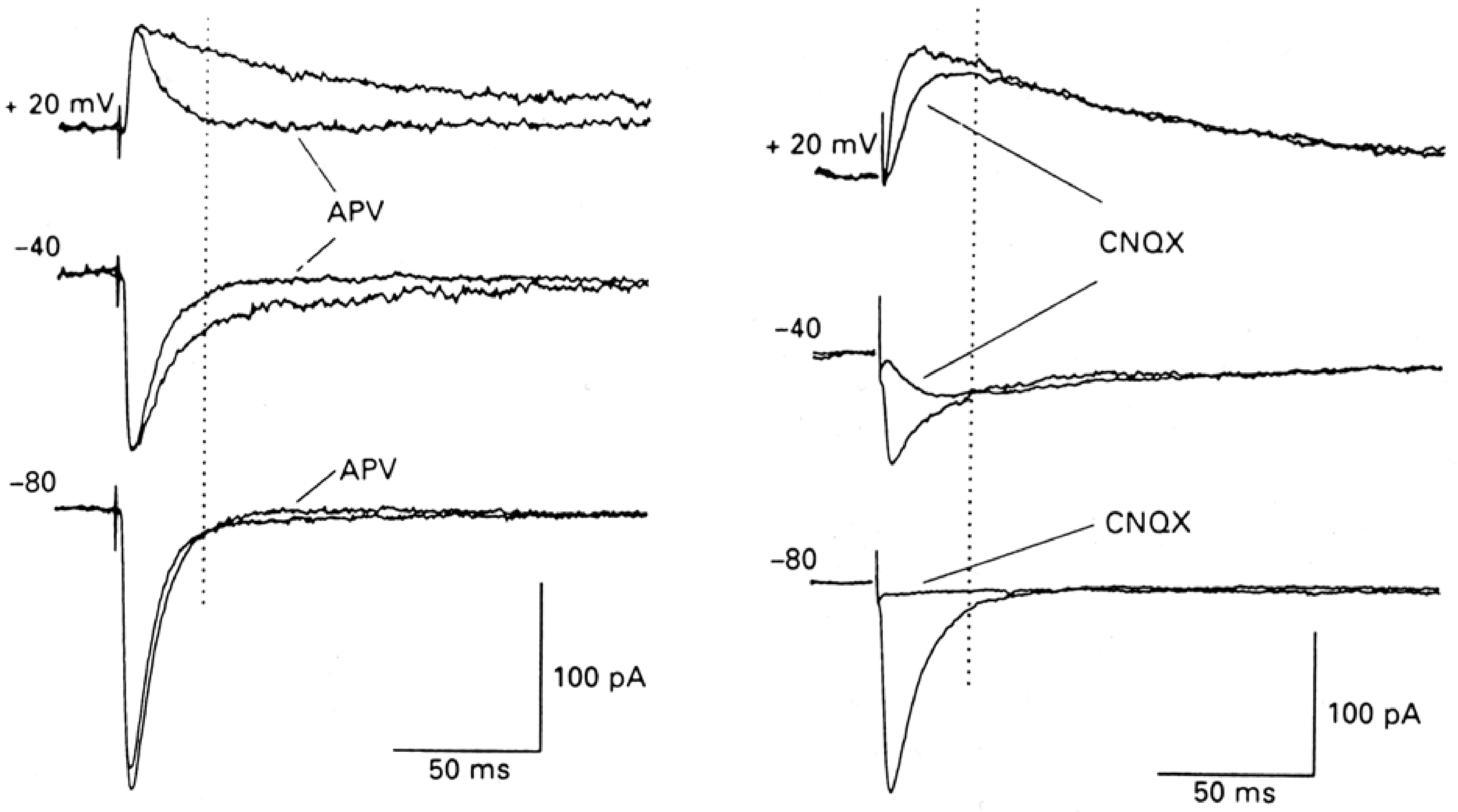
Figure 4 APV blocks the NMDA receptors leaving only the AMPA receptors (left panel). CNQX blocks the AMPA receptors leaving only the NMDA receptors (right panel). Note that at typical resting membrane potentials (-80 mV) there is almost no NMDA receptor mediated current.
APV has very little effect on synaptic transmission at -80 mV but has a large effect at +20 mV when the NMDA receptors are making a significant contribution to the synaptic current. CNQX eliminate the AMPA receptor current leaving the NMDA receptor current intact, illustrating the slow activation as well as the slow inactivation of the NMDA receptor, even though both receptors see the same changes in glutamate in the synaptic cleft. The AMPA receptor current decays much faster (≈6 ms) than the NMDA receptor (≈100 ms). Neither decay time is dependent on voltage.
The relative ratio of NMDA receptors to AMPA receptors at different glutaminergic synapses is quite variable. Typically, this is due to a wide variation in the number of AMPA receptors at the synapses due to the ongoing regulation of AMPA receptor expression by electrical activity.
Long term potentiation
The strength of synaptic connections in the CNS are strongly dependent on the prior level of electrical activity at the synapse. This general phenomenon is known as synaptic plasticity. Long-term potentiation (LTP) is a form of synaptic plasticity that occurs at glutaminergic synapses.
If the nerve fibers innervating a neuron are stimulated at a low frequency, there is a baseline level of synaptic input that is essentially unchanging (Figure 5C). If the nerve is rapidly stimulated for a brief period of time, known as tetanic stimulation, two changes in synaptic transmission occur. Following the tetanus there is a transient increase in synaptic strength known as post-tetanic potentiation (PTP). PTP is found at most synapses and its duration is typically a few minutes. PTP is due to a transient increase in the concentration of calcium in the nerve terminal that enhances transmitter release following the tetanus. After the tetanus the calcium concentration in the nerve terminal gradually falls and transmission returns to normal levels at many synapses. LTP is a maintained enhancement of synaptic transmission that can last for up to a week in intact animals depending on the duration and number of tetanic stimuli.
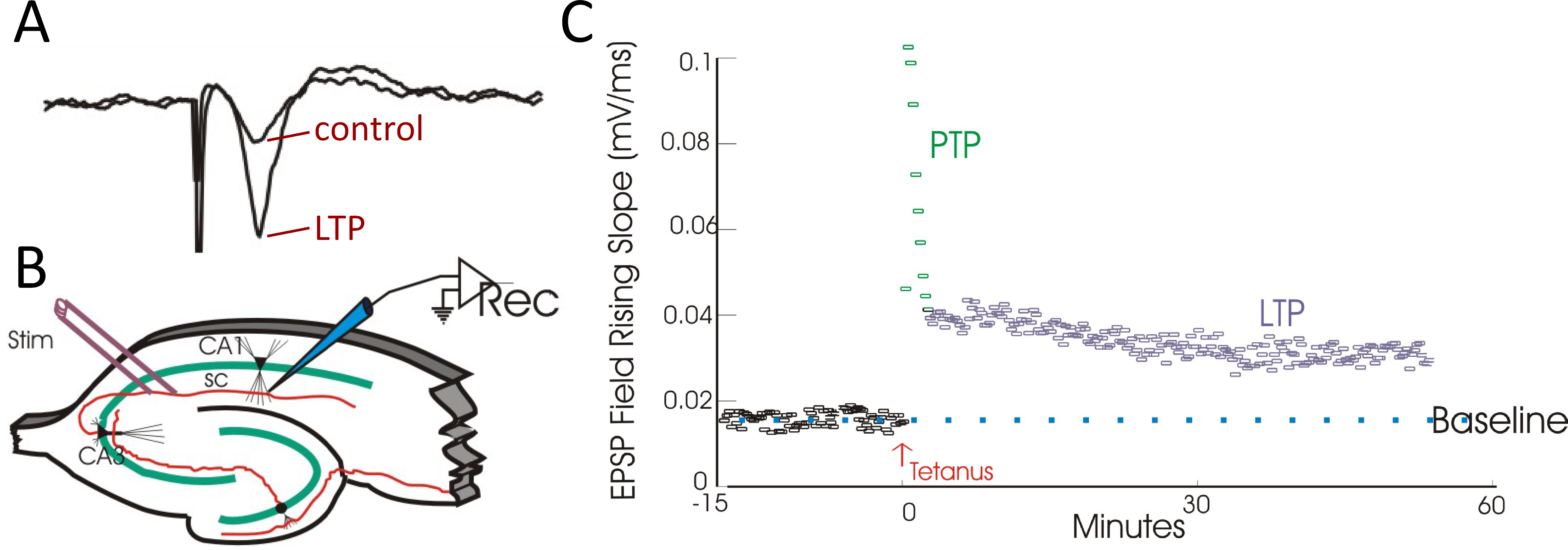
Figure 5 A. Field potential recordings of excitatory synaptic transmission before (control) and after tetanic stimulation (LTP). B. Recording setup in hippocampal slice. Schaffer collaterals (SC) were stimulated and recordings were made in CA1. C. Following tetanus there is a short period of enhanced synaptic transmission known as post-tetanic potentiation (PTP) followed by a long, sustained enhancement known as long-term potentiation (LTP) (right panel).
The recordings shown in Figure 5A measure LTP in a population of cells. The recording electrode is placed amongst a group of cells and it records what is known as a field potential, which reflects the current flow produced by epscs occurring at a large number of synapses. This way of recording, because it averages across many synapses, tends to be a more reliable way to examine LTP than recording single synaptic inputs.
Properties of LTP
LTP has three key properties (Figure 6):
1. Specificity - When a strong input (a relatively large number of fibers) is stimulated it will display LTP but synapses in other regions of the neuron will not. Inputs that are not active at the time of the tetanus of the strong input do not show potentiation.
2. Cooperativity - If only a weak input (relatively few fibers) is stimulated, LTP does not develop. It is only when a relatively large number of inputs are stimulated that there is induction of LTP. There is a threshold for induction of LTP.
3. Associativity - If a strong input is paired with a weak input, then the weak input will display LTP, even though the weak input by itself cannot induce LTP.
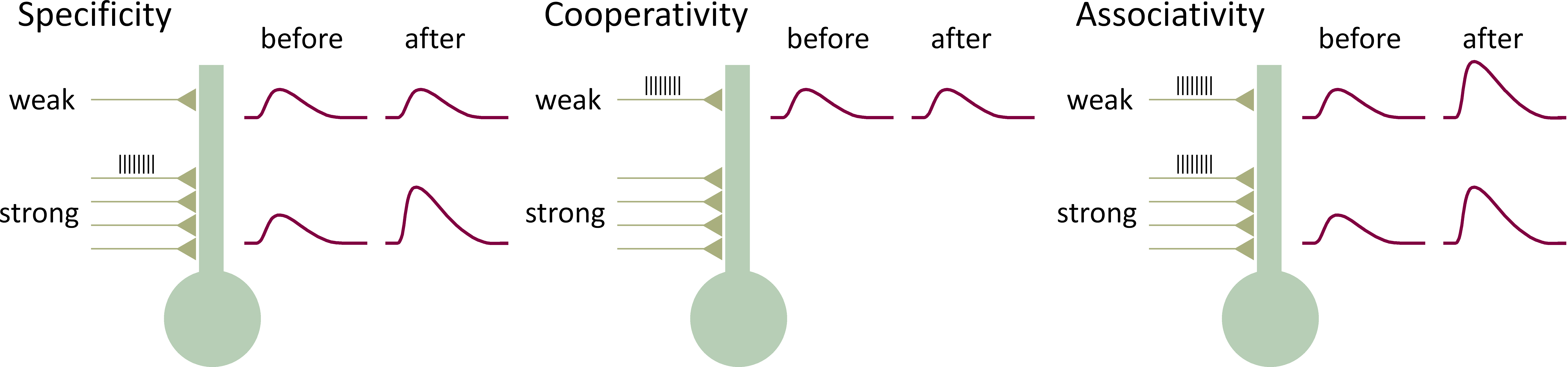
Figure 6 Properties of LTP. The figure illustrates the effect of stimulating both a strong input and/or a weak input to the neuron.
The properties of LTP can be explained by the fact that depolarization of the postsynaptic cell is required for the induction of LTP. If depolarization of the postsynaptic cell by synaptic input is blocked by voltage-clamping the cell, then LTP does not occur. Conversely, if a weak input is paired with an experimentally induced depolarization, using current injection into the cell, then the weak stimulus will elicit LTP, even though it would not do so in the absence of the depolarizing current. The depolarizing current is sufficient to replace the strong paired synaptic stimulus, which is required in associativity.
These synapses are known as Hebbian synapses because of the requirement of post-synaptic activity in conjunction with presynaptic activity in order to evoke strengthening of the synapse. This requirement was originally proposed by Canadian psychologist Donald Hebb as a mechanism for synaptic learning. Modulation of the strength of synaptic connections is also critical during development for establishing the correct synaptic connections.
Calcium Influx is Required for LTP
At normal resting membrane potential, the NMDA receptor is blocked by magnesium ions, which sit in the channel (Figure 7). To open the NMDA receptor two things must happen. First the neurotransmitter glutamate must bind to the receptor. Second, the cell membrane must be depolarized in order to relieve the magnesium block.
The NMDA receptor is doubly gated both by the agonist glutamate and by voltage, which acts to relieve the magnesium block. The receptor acts as a coincidence detector. It only opens when there is a coincidence of synaptic input and postsynaptic depolarization. When the NMDA receptor channel is both activated by glutamate and depolarized, relieved of Mg2+ block, then Ca2+ ions can flow into the cell, which is the essential first step for the induction of LTP.
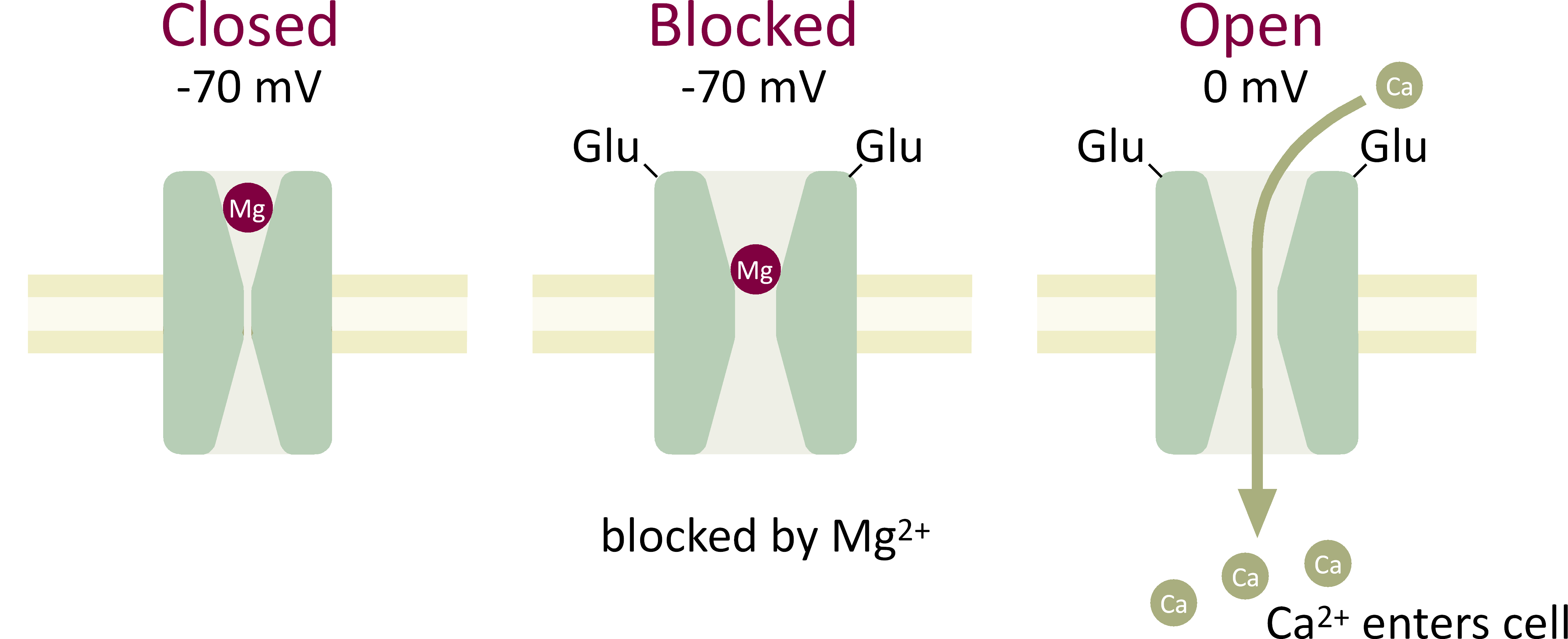
Figure 7 Double gating of the NMDA receptor by glutamate (Glu) and depolarization.
The key role of Ca2+ influx is demonstrated by injecting the Ca2+ chelating agent BAPTA into the neuron. In the presence of BAPTA the Ca2+ concentration does not rise significantly because all the Ca2+ influx is mopped up by the buffer. Under these conditions LTP is not induced, even during a strong synaptic input. Similarly, APV can block the formation of LTP due to blockade of glutamate binding to the NMDA receptor and subsequent activation of Ca2+ influx through the receptor’s channel.
The property of specificity (Figure 6) is explained by the fact that only the strong inputs depolarize the cell sufficiently to relieve the Mg2+ block of the NMDA receptor and allow a Ca2+ influx. Cooperativity occurs because a strong synaptic input is required in order to depolarize the cell to produce a Ca2+ influx. Associativity occurs because the strong input can depolarize the cell sufficiently to allow Ca2+ influx at the synapses innervated by the weak input.
Regulation of AMPA Receptor Expression
The level of AMPA receptor expression at individual synapses is tightly regulated. Associative LTP works predominantly via the upregulation of AMPA receptor expression at specific synapses. Calcium influx via calmodulin activates the kinase CaMKII located at the synapse. This, in turn, triggers transport of AMPA receptors to the post-synaptic density.
Other Forms of LTP
Associative, or Hebbian, LTP described above is not the only form of LTP found in the brain. Hebbian LTP is NMDA receptor dependent. It is called associative because a strong input can result in the enhancement of a weak input thereby creating an association between the two inputs if they occur together in time. This form of LTP provides a mechanism for encoding associations between temporally correlated events.
Other forms of LTP that are not associative and do not require NMDA receptors have also been described. Non-Hebbian LTP was first well characterized at the synapses between the mossy fibers and the CA3 neurons. These synapses have few or no NMDA receptors. Formation of LTP is not dependent on post-synaptic depolarization and appears to be primarily a presynaptic event. Because depolarization of the post-synaptic cell is unimportant, strong inputs cannot enhance weak inputs.
Long-Term Depression (LTD)
In addition to being strengthened synapses can also be weakened. These balancing effects are important to maintaining a stable level of synaptic input to a given neuron. One mechanism by which weakening of synaptic contacts occurs is Long-Term Depression (LTD). LTD can be triggered in CA1 neurons of the hippocampus by persistent weak synaptic stimulation and it involves a reduction of AMPA receptor expression
Excitotoxicity
Calcium influx through NMDA receptors can also have very negative consequences. During strokes, when the blood flow is blocked to parts of the brain, the nerve terminals can depolarize releasing glutamate. The glutamate activates NMDA receptors allowing Ca2+ ions to flow into the postsynaptic neuron. The resulting rise in internal Ca2+ concentrations can kill the postsynaptic cell. This phenomenon is known as excitotoxicity, because cell toxicity is produced by excitatory neurotransmitters.