G Protein-Coupled Receptors
The G protein-coupled receptors generally share a common structure; they have seven membrane spanning regions and the agonist binding site lies in a pocket between the membrane spanning domains (Figure 1). These receptors do not contain an integral ion channel. Instead, they act indirectly, generally via intermediary proteins, on effector proteins that can be, but are not necessarily, ion channels.
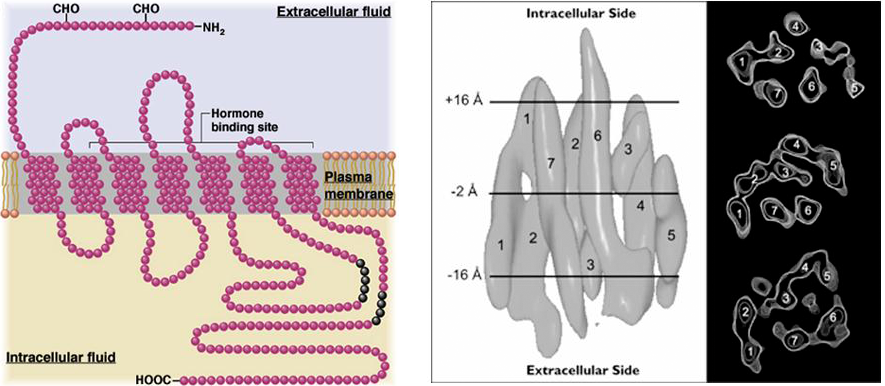
Figure 1 Membrane topology of G protein-coupled receptors, with the amino and carboxy termini located extracellularly and intracellularly respectively and the seven membrane spanning domains (left panel). Low resolution structure showing the membrane spanning regions within the membrane (right panel). The ligand binding site is typically in a pocket within the structure. Note the relatively small size of the receptor compared to the ligand-gated ion channels.
Most of the actions of G protein-coupled receptors are mediated via intermediary proteins known as G-proteins. The G-proteins are peripheral membrane proteins located on the intracellular surface of the membrane. The G-proteins are heteromultimers, comprised of three different subunits: α, β, γ. The α-subunit binds the nucleotide GTP (hence the name G-protein). The three subunits are encoded by more than 100 different genes and the different combinations of subunits that this allows provides for the specificity of effect following receptor activation i.e., different G-proteins bind to different receptors and target different effector molecules.
The basic mechanism by which G protein-coupled receptors work is shown in Figure 2. At the core of this mechanism is the fact that the α-subunit is a GTPase, albeit a rather inefficient one. In each passage of the cycle one GTP molecule is converted to GDP. When ligand binds to the receptor, the α-subunit of the G-protein releases GDP and binds GTP. Binding of GTP induces a conformational change and the trimeric G-protein separates into two parts, the α-subunit and the β/γ subunits. These two components can then activate or inhibit effector proteins independently. The cycle ends when the α-subunit hydrolyzes GTP to GDP and the α-subunit rebinds to the β/γ subunits, thereby inactivating both components of the G-protein. The efficiency of the GTPase determines how long the system is turned on. Typical values are of the order of one minute, a relatively long-lasting effect.
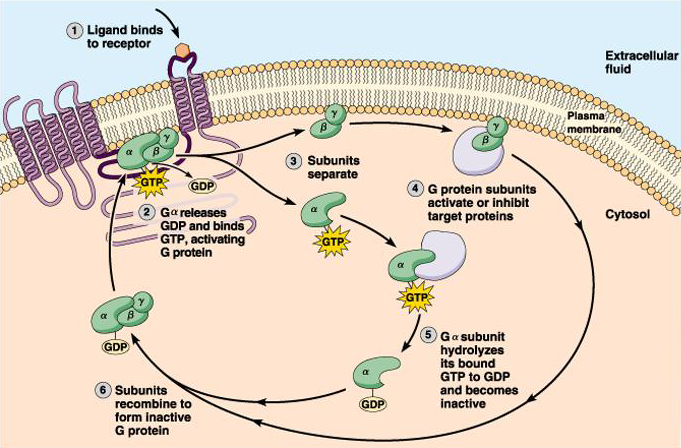
Figure 2 Cycle of G protein activation following receptor stimulation.
The effector proteins that can be stimulated or inhibited by the G-protein subunits are a remarkably diverse set of proteins. Typically, a subunit of the G-protein can directly activate or inhibit an ion channel, or it can activate an enzyme that produces a second messenger that activates a second effector protein, such as a kinase, that can then modify the function of an ion channel. Channels are not the only targets of G-proteins however, and activation of G-protein receptors can modulate almost any cellular function.