Cys-loop Receptors
The Cys-loop receptor family encompasses a broad range of receptors, including the nicotinic acetylcholine, GABAA, glycine and 5-HT3 receptors (Table 3). The name of this class derives from a shared structural feature, a loop formed by a disulfide bond between two cysteine residues found in the N terminal extracellular domain of the receptor.
There are two main subfamilies, cation selective and anion selective channels. This is a key distinction physiologically, since the cation channels are excitatory in function, they depolarize the membrane potential, whereas the anion channels are generally inhibitory in function.
Table 1 Cys-loop Receptors
| A. Cation Channels | |
|---|---|
| nicotinic acetylcholine receptors | 16 genes |
| serotonin 5-HT3 receptors | 5 genes |
| B. Anion Channels | |
|---|---|
| GABAA receptors | 19 genes |
| glycine receptors | 5 genes |
Cys-loop Receptor Structure
The prototypical Cys-loop receptor is the acetylcholine receptor expressed in skeletal muscle. It is a pentamer comprised of four different subunits: α, β, γ, δ, with the stoichiometry: α2βγδ. Each of these subunits has four membrane spanning domains. The five subunits assemble in the receptor to form a pseudo-fivefold symmetry, with each subunit contributing to the formation of the pore (Figure 1).
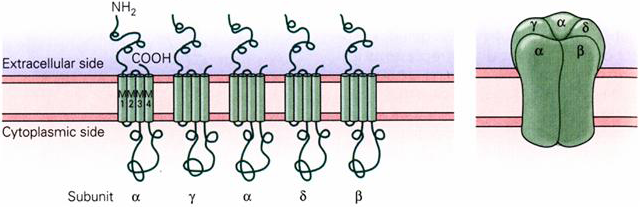
Figure 1 Structure of the neuromuscular nicotinic acetylcholine receptor
The subunits have three main functional domains: the ligand binding (LBD), transmembrane (TMD) and intracellular domains (IND) (Figure 2). The receptor has two agonist binding sites that reside primarily in the α-subunits and are found at the interface between each of the two α-subunits and the neighboring γ and δ subunits. The two binding sites have different affinities for acetylcholine. A striking feature of the receptor is how much of it projects out of the plane of the cell membrane (Figure 2A). The extracellular region of the receptor projects more than 65Å into the extracellular space. The agonist binding site is located a considerable distance (50Å) from the gate of the channel, which is located in the middle of the membrane spanning domain. The intracellular surface projects 20Å into the intracellular space. The large blob that hangs from the base of the receptor is comprised partly of receptor protein but also contains a clustering protein (rapsyn). There are large tunnels between the blob and the main part of the receptor that allow ion movement into the channel.
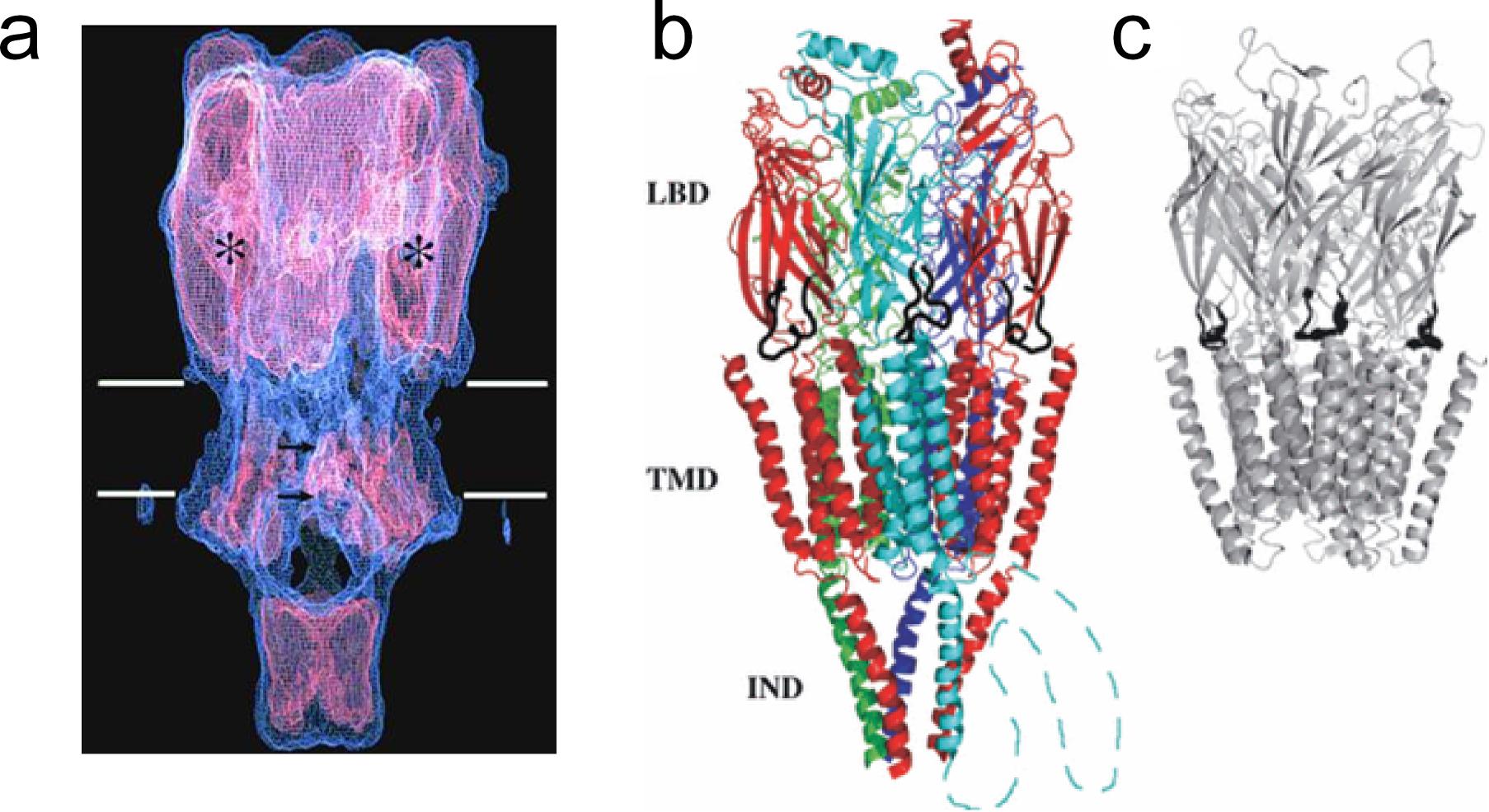
Figure 2 A. Low resolution structure of the nicotinic acetylcholine receptor. The two white lines indicate the boundaries of the cell membrane. The ACh binding pockets (asterisks), gate of the channel (upper arrow) and selectivity filter of the open channel (lower arrow) are marked. The subunits are slightly tilted around the axis of the receptor. The ion conduction pathway goes through tunnels between the large intracellular domain at the base of the receptor and the main part of the receptor. B. High resolution structure of the nicotinic acetylcholine receptor. Each of the five different subunits has a different color. The ligand binding (LBD), trans-membrane (TMD) and intracellular domains (IND) are marked. C. High resolution structure of the prokaryotic Cys-loop receptor. The Cys-loops of the receptors are marked in black in (b) and (c). Only the three front loops can be seen.
The cys-loop receptors are evolutionary ancient. A simpler form of the receptor can be found in prokaryotes (Figure 2C), where it appears to function as a chemoreceptor. A high-resolution structure of the nicotinic acetylcholine receptor found in muscle cells is shown in Figure 3.
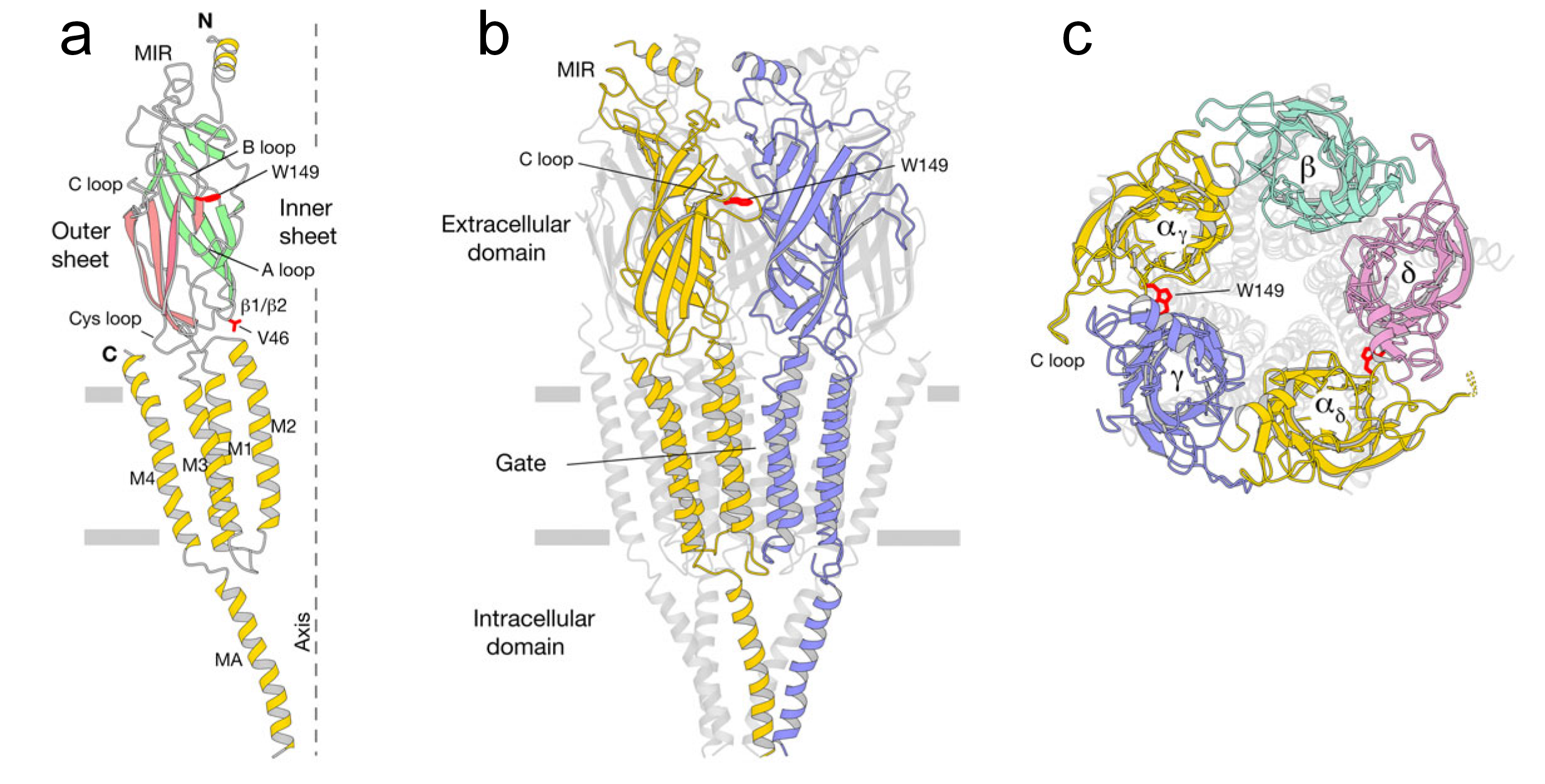
Figure 3 Nicotinic acetylcholine receptor. A. Alpha subunit. B. Side view of receptor. C. Top view. Marked in red are the sidechains of the ACh-binding residues αW149 on the B loop and αV46 on the β1/β2 loop.
Ion Permeation through the Acetylcholine Receptor
The four membrane spanning domains of each subunit are named M1 through M4 (Figure 4). The M2 domain lines the inner surface of the channel and the other helices are arranged between this helix and the lipid bilayer. The M2 helix is the primary determinant of the ion permeation properties of the channel.
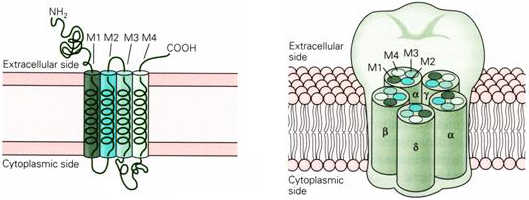
Figure 4 Location of M2 membrane spanning region within the acetylcholine receptor.
The skeletal muscle acetylcholine receptor has similar permeability to Na+ ions as it has to K+ ions, but does not allow Cl- ions to pass. The structure of the pore is less specialized than that of the K+ ion selective channels described previously because it only has to distinguish between positively and negatively charged ions, not between ions with the same charge but of different size.
Charged residues within the channel pore play a major role in determining the ion selectivity of the pore. There are three rings of negatively charged amino acid side chains within the pore (Figure 5). These rings contain three or four negative charges. The external and inner rings act to decrease the local concentration of anions around the entrance to the pore. The selectivity filter is most directly associated with the intermediate ring of negative charges on the cytoplasmic surface of the channel.
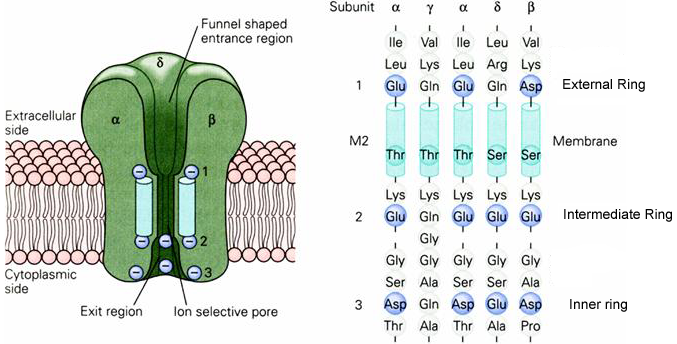
Figure 5 Location of three rings of negatively charged side chains within the channel pore. Notice that immediately before the selectivity filter (intermediate ring) is a positively charged lysine residue. This positive charge is normally turned away from the pore.
The intermediate ring is the region where the pore is at its narrowest in the open state. The pore is physically quite a bit larger than the K+ channel pore and the ions do not completely lose their hydration shell during their passage through the channel. Selectivity is produced by electrostatic interactions between the ions and charged side chains.
The central role of the intermediate ring in determining ion selectivity is shown by experimental manipulation of the charges in these side chains. Although something of a simplification, a cation selective channel can be converted to an anion selective channel by changing the intermediate ring from a negatively charged ring to a positively charged ring.
The structure of the cation selective AChR and the anion selective Glycine receptor are quite similar. A key difference lies within the pore sequences of the two receptors (Figure 6). Just two mutations can convert the cation channel to an anion channel and vice versa; the conversion of the intermediate negative ring to uncharged alanine and the addition of a proline residue, which twists the positively charged arginine to face into the pore. All GABA and Glycine receptors have this proline residue. This experiment shows that the ion selectivity of the channel largely depends upon electrostatic interactions between the ions and the charged residues in this intermediate ring.
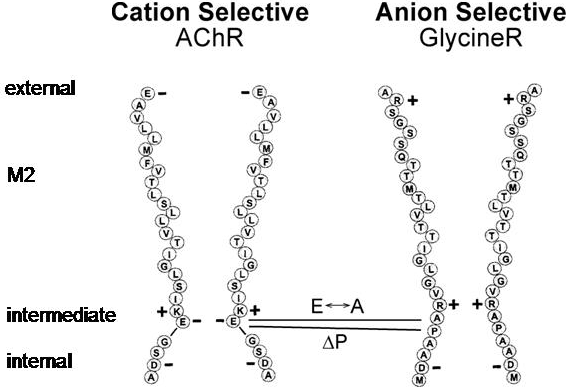
Figure 6 Comparison of the M2 pore lining regions of the acetylcholine receptor and the glycine receptor.
Although mutations in the intermediate ring largely determine whether or not the channel is an anion or a cation channel this does not mean that other regions of the pore are unimportant. Negative charges in the other rings act to increase the local concentration of cations, which increases the single channel conductance of the channel.
Gating of the Acetylcholine Receptor
Acetylcholine binds to two pockets that are relatively deep and provide multiple contact sites between the ligand (ACh) and the receptor ensuring a high degree of binding specificity (Figure 7).
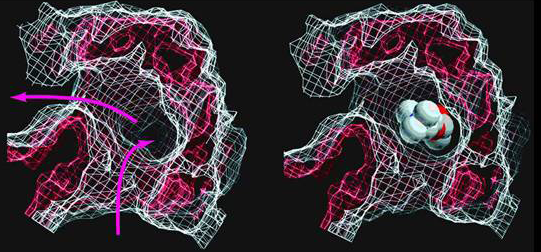
Figure 7 High resolution image of the ACh binding pocket in the alpha subunit. Figure shows the pocket with (right) and without ACh.
It is the binding of acetylcholine to the receptor that induces a conformational change in the receptor which leads to channel opening. Acetylcholine binding produces rearrangements in the regions of the protein surrounding the agonist binding site (Figure 8). Gating of the channel is dependent on the energy associated with acetylcholine binding. This is in contrast to voltage-gated ion channels where changes in the membrane potential provide the energy for the conformational change that produces gating.
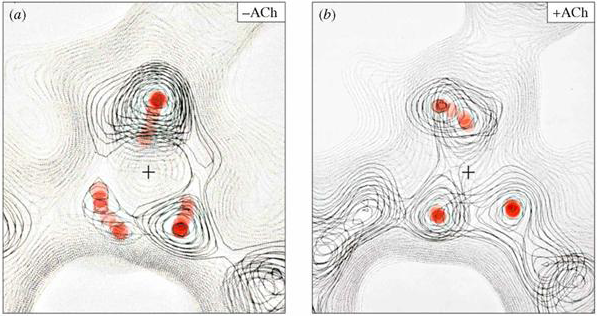
Figure 8 Changes in the local structure of the receptor following ACh binding. Note that the rearrangement of the protein around the binding site (marked with red dots) following ACh binding.
The gate in the closed channel is formed by the hydrophobic region of the M2 helix (Figure 9). The leucine and valine side chains form a hydrophobic ring that closes the channel by making an energy barrier that hydrated ions cannot cross.
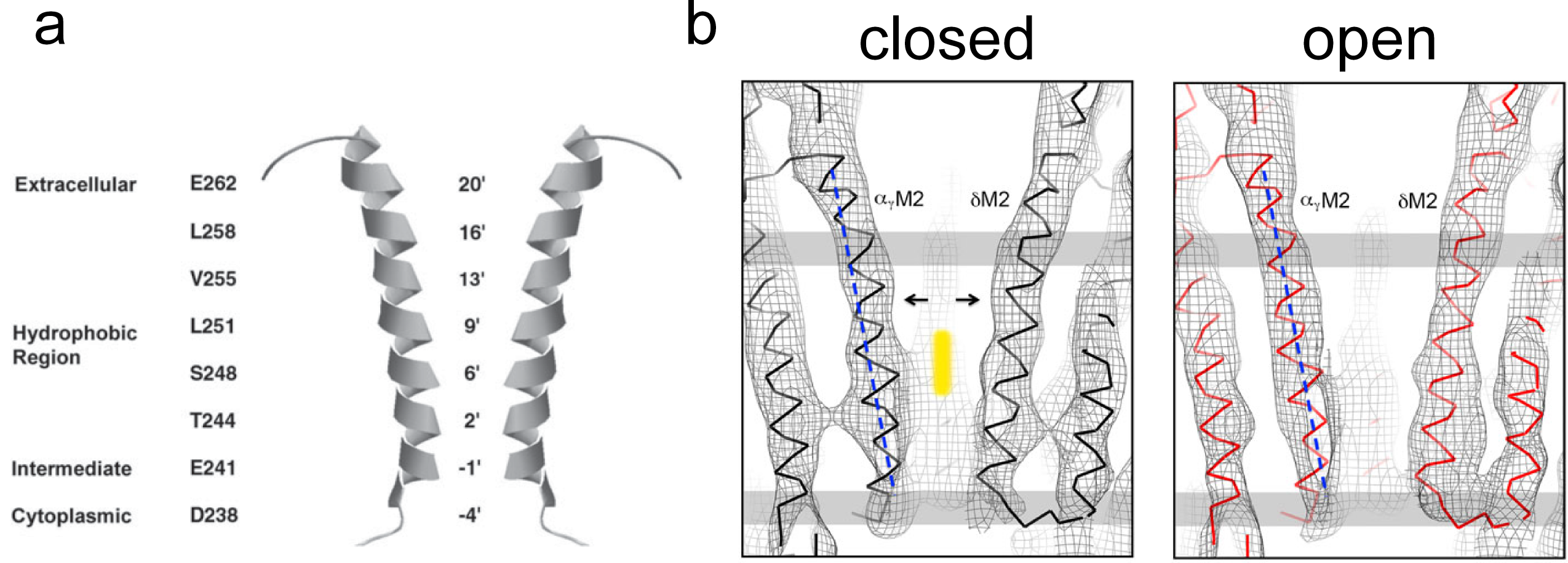
Figure 9 A. M2 channel lining residues. B. Structure of the pore in the closed and open confor-mations. The vertical yellow bar in identifies the location of the gate, near the middle of the membrane (grey bars).
Opening of the channel by ACh binding is a result of widening of the hydrophobic region of the pore (Figure 9B). At this point the channel becomes a water-filled pore allowing movement of ions across the membrane. The structural changes are relatively subtle but produce a significant change in the energy landscape for ion permeation through the channel.