Ion Channel Permeation and Selectivity
Ion channels are membrane proteins that form continuous channels from one side of the membrane to the other through which ions can pass into or out of the cell. All ion channels have two key properties: ion permeation, they provide a pathway for ions to permeate across the membrane, and ion selectivity, they allow some ions to pass more easily than others. These properties are intimately related and will be discussed together.
Most ion channels have a third property known as gating. Gating means the opening and closing of the pore of the ion channel. The gating mechanism shows marked differences between different channels. Some aspects of gating will be described in the final section of this chapter.
The crystal structure of several K+ channels has been solved and ion permeation, selectivity and gating are now well understood for this channel. For this reason, much of this discussion will focus on K+ channels.
Potassium Channels
Typically, the pores of K+ channels are highly selective, favoring K+ ions over Na+ ions by a ratio of 10,000:1. Potassium channels are also very efficient, allowing approximately 108 ions to pass per second. Essentially, K+ ions can pass through the channel at the same rate at which they arrive at the mouth of the channel. Energetically, the channel appears to be almost transparent to the ions as they pass through it.
Quaternary Structure of K+ Channels
Most K+ channels are tetramers, meaning that they are assembled from four protein subunits. The simplest K+ channel subunits have two membrane spanning domains (Figure 1A). The amino- and carboxyl-terminals of these subunits are located on the cytoplasmic side of the membrane. The four subunits co-assemble around a central ion pore something like the staves of a barrel (Figure 1B).
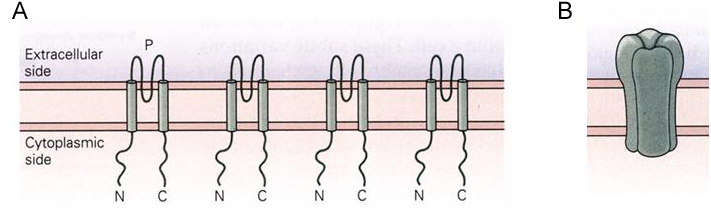
Figure 1 (A) K+ channel subunits with two membrane spanning domains. (B) Subunits assembled into a channel.
The crystal structure for a simple K+ channel is shown in Figure 2. The channel has three prominent alpha helices per subunit: an inner and outer helix, which are both membrane spanning, and a pore helix located near the extracellular surface of the channel. The inner helix is more closely associated with the channel pore than the outer helix.
This simple form of the K+ channel is a relatively small protein that sits largely within the boundaries of the membrane’s lipid bilayer (Figure 2C)
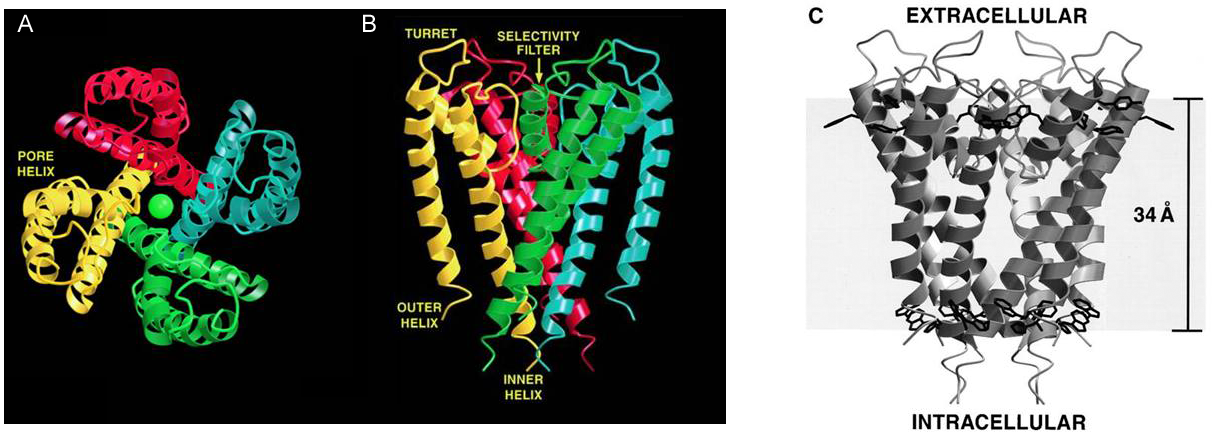
Figure 2 Crystal structure of a simple K+ channel. (A) The four subunits of a K+ channel arranged around a central pore. Each subunit is shown in a different color and a K+ ion (green ball) is located within the pore. (B) Side view of the channel showing the two membrane spanning domains of each subunit. (C) Location of channel protein within the lipid bilayer of the membrane.
Quaternary Structure of K+ Channels
The pore is 45 Å long and has a narrow selectivity filter close to the extracellular surface, with a relatively wide water-filled pore connecting this to the intracellular surface (Figure 3). Potassium ions can traverse from the intracellular solution through the internal pore to the selectivity filter while retaining their hydration shell. Essentially, they remain in an aqueous environment until they enter the selectivity filter, which is much narrower.
In principle, the primary energy barrier for ion movement will be greatest at the center of the channel where it should be most difficult for the ion to polarize the surrounding channel protein due to the hydrophobic nature of the protein in the lipid bilayer. The channel solves this problem by surrounding the ion with polarizable water molecules for much of its journey through the channel. In addition, the pore helices are positioned to maximize the helix-dipole effect, allowing the protein itself to be polarized by the ion. Essentially the cavity acts as a well of polarizable water, and ions can move freely from the intracellular solution to the cavity and back out again.
The selectivity filter is much narrower, however, and the ion must shed most of its hydration shell in order to pass through the filter.
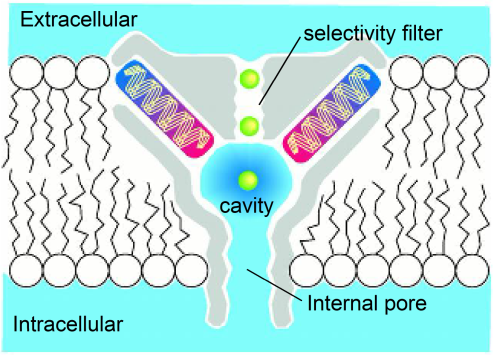
Figure 3 Cartoon showing the pore domains, the long internal pore leading to the cavity adjacent to the narrow selectivity filter that separates the water filled pore from the extracellular solution.
Structure of the Selectivity Filter
The amino acid sequence of the selectivity filter is conserved in all known types of potassium channels from bacterial potassium channels to plant and animal channels. During the course of evolution, a mechanism to selectively catalyze the movement of K+ ions across the cell membrane has evolved only once.
The conserved amino acid sequence of the selectivity filter is (T/S)(V/I)G(Y/F)G. The last three residues GYG are conserved in almost every potassium channel, with only a few rare exceptions having the alternate GFG sequence. This short sequence of amino acids lines the surface of the selectivity filter (Figure 4).
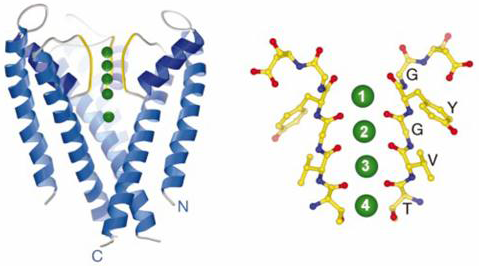
Figure 4 (left panel) The K+ channel with the front subunit removed. The yellow region corresponds to the selectivity filter and the green spheres correspond to K+ ions. (right panel) High resolution image of the selectivity filter showing the TVGYG amino acid sequence. Only two of the four subunits are shown. Each red dot corresponds to a carbonyl oxygen atom. There are five rings of carbonyl oxygen atoms each containing four oxygen atoms, one from each subunit.
The structure of the TVGYG sequence is highly unusual. The amino acids are arranged to form an almost straight line running up the sides of the pore and the side chains of the residues are turned away from the pore so that the closest contact point between the ion and the channel protein is with the carbonyl oxygen atoms, which are part of the peptide backbone. It is the arrangement of these carbonyl oxygen atoms that is critical to understanding the function of the pore.
In solution, K+ ions are surrounded by a tight shell of water molecules, known as the inner hydration shell. The oxygen atoms of these water molecules are oriented to face the positively charged K+ ion. The selectivity filter replaces the shell of oxygen atoms provided by the water molecules with the rings of carbonyl oxygen atoms. These five rings of oxygen atoms replicate the position of the oxygen atoms of the water molecules of the inner hydration shell. In this way the channel mimics the normal aqueous environment of the K+ ions and there is almost no energy barrier for the movement of the K+ ions from the aqueous solution into the selectivity filter and back out again.
Ion Selectivity
The structure of the selectivity filter explains in a very simple and elegant way how the K+ channel can show such great selectivity for K+ ions over Na+ ions.
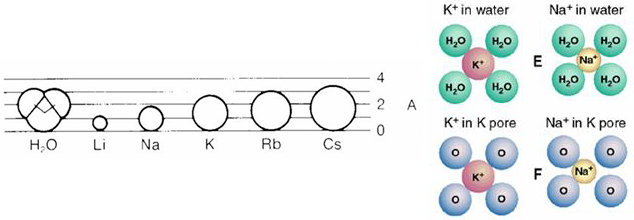
Figure 5 (left panel) Comparison of the ionic radii of different alkali-metal atoms. (right panel) The relationship between oxygen atoms of water molecules and K+ and Na+ ions and between oxygen atoms of the selectivity filter and K+ and Na+ ions.
There is relatively little difference between a K+ ion and a Na+ ion. They have the same charge and relatively similar ionic radiuses: K+ 1.33 Å and Na+ 0.95 Å (Figure 5). In solution, the oxygen atoms of the water molecules move freely and can adjust to the different ionic radiuses so that both atoms can easily dissolve in an aqueous environment. In the selectivity filter of the K+ channel, the oxygen atoms are held in a relatively rigid spatial arrangement into which the K+ ion fits well but the Na+ ion less well (Figure 5). This relatively small geometrical difference translates into a large difference in the ease with which the two ions can move into the selectivity filter. Potassium ions partition easily into the filter whereas sodium ions cannot move as readily into the selectivity filter. This difference in the energy state of the two ions in the pore is the source of the differential selectivity of the channel to the two ions.
Ion Permeation
The selectivity filter has five oxygen rings that result in four binding sites for ions within the filter (each binding site is made up of two rings of four oxygen atoms). Because the K+ ions are charged, adjacent binding sites cannot be occupied due to electrostatic repulsion between the two adjacent K+ ions. As a consequence, the channel cycles between two semi-stables states when it is conducting ions (Figure 6). The difference between these two states is very subtle and does not involve significant rearrangements of the channel protein. As a consequence, cycling between the two states is very rapid and ions move through the channel very efficiently.
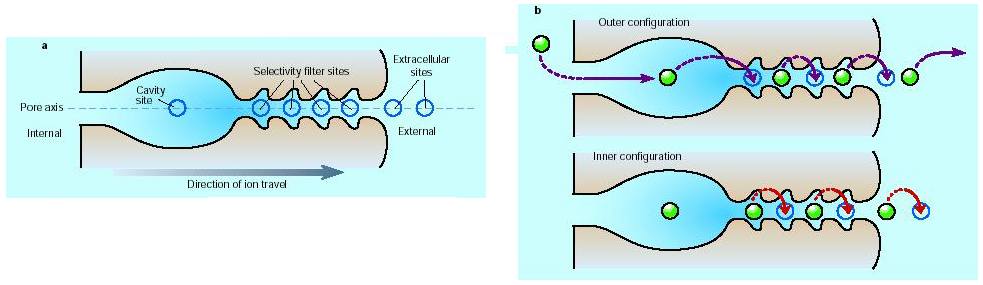
Figure 6 (A) Four binding sites for K+ ions within the filter. (B) Two semi-stable states for ions in the filter. Ions move along the channel by jumping from one binding site to the next. Electrostatic repulsion by new ions entering the cavity pushes the chain of ions through the filter.
The constant arrival of new ions in the cavity acts to push ions out of the other end of the filter due to the electrostatic repulsion between the ions within the selectivity filter.