Electrically Excitable Cells
For multicellular animals the electrically excitable cells are those cells that can mediate an action potential, primarily the neurons and muscle cells. An action potential is an electrical event across the cell membrane that is triggered by a depolarizing current stimulus (Figure 1).
Figure 1 Stimulation of an action potential in an electrically excitable model cell. Press the ‘+’ button to inject a depolarizing current into the cell. The membrane potential (top panel), the state of the voltage-dependent sodium conductance (orange) and potassium conductance (green) (middle panel), and the injected current stimulus (bottom panel) are shown.
If a small current is injected into an excitable cell, the membrane behaves like the parallel RC circuit described previously. This is known as the passive electrical response. At first the voltage response increases linearly with linear increases in the amount of the injected current, as expected from Ohm’s law. In the model cell, for current steps up to 2.5 nA, the voltage response increases linearly as expected. Larger currents, however, trigger an action potential. This is known as an active response because it is non-linear.
During an action potential the electric potential across the cell membrane undergoes a rapid change from the resting voltage, approximately -76 mV, to a positive voltage, about +45 mV, and then repolarizes back to the resting voltage. The rising and falling phases of the action potential are completed relatively quickly, taking approximately 1 ms. It takes longer for the system to completely return to the resting state. In this particular cell there is a long afterhyperpolarization where the membrane potential stays below the resting membrane potential.
The state of the two voltage-dependent conductances in the membrane, which are mediated by voltage-activated sodium and potassium channels, changes during the action potential. These conductances quickly increase before returning more slowly to their resting state. The changes in these two conductances are what drives the changes in membrane voltage. The time course and magnitude of the changes in membrane voltage and conductance are very similar when an action potential is triggered by differently sized depolarizing currents.
Description of the Action Potential
The following terminology is commonly used to describe action potentials.
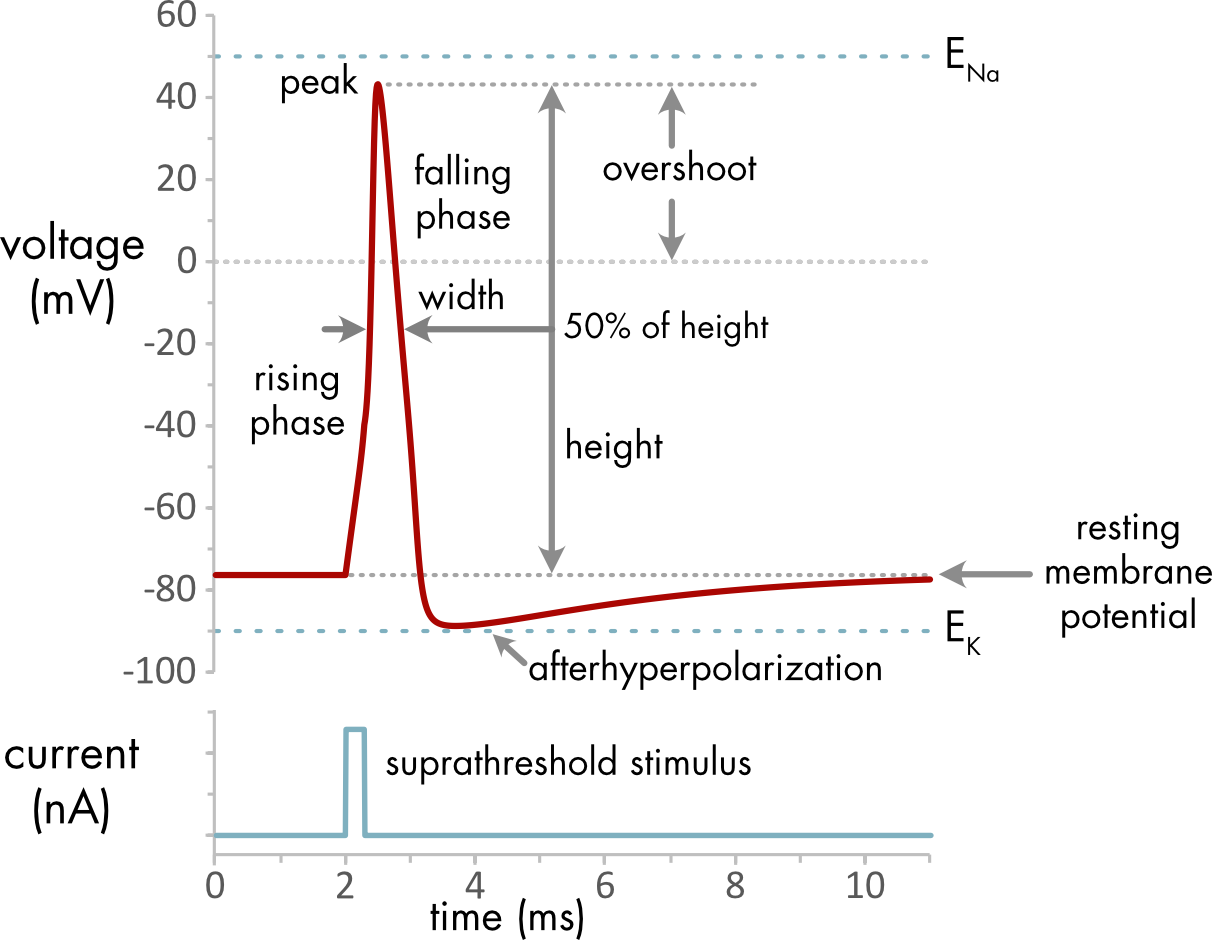
Figure 2 Action potential properties.
| Action Potential Properties |
|---|
| resting membrane potential: electric potential across the cell membrane in the absence of any current stimulus |
| rising phase: first phase of the action potential, membrane potential moves rapidly towards a positive potential |
| peak: the maximum voltage during the action potential |
| falling phase: second phase of the action potential, membrane potential moves rapidly towards more negative potentials |
| afterhyperpolarization: third phase of the action potential, membrane potential typically falls below the resting potential |
| action potential height: difference in voltage between the resting membrane potential and the peak |
| width: width of the action potential, typically measured at 50% of spike height |
| overshoot: how far the membrane potential goes positive relative to 0 mV |
| suprathreshold stimulus: a depolarizing current stimulus that is large enough to trigger an action potential |
During an action potential the voltage remains constrained between the equilibrium potentials for potassium ions (\(E_{K}\)) and sodium ion (\(E_{\mathit{Na}}\)), since there is normally no driving force to move the voltage outside that range. At the peak of the action potential the voltage approaches \(E_{\mathit{Na}}\), as the sodium conductance becomes relatively large. During the afterhyperpolarization the membrane voltage approaches more closely to \(E_{K}\), because the potassium conductance is larger at this point than at rest.
The rising phase is similar in most excitable cells, driven by a large increase in the sodium conductance. Action potential width varies in different mammalian neurons over a range of approximately 0.5 to 5 ms. It is much longer in cardiac muscle cells, up to 300 ms. The rate of voltage change during the falling phase depends primarily on the number and kinetics of the voltage-gated potassium channels, which varies considerably between different excitable cell types. The afterhyperpolarization is also very variable among different neuron types. It can vary from a few milliseconds, up to several hundred milliseconds. In some neurons there is instead an afterdepolarization, where the membrane remains positive to the resting membrane potential for a period following the action potential. These differences depend on which different ion channel genes are expressed in the cell, which varies considerably between different cell types.
A suprathreshold stimulus is a current stimulus large enough to trigger an action potential. Subthreshold current stimuli do not trigger an action potential. A threshold stimulus is a current that is just large enough to trigger an action potential. This value depends on the balance of depolarizing and hyperpolarizing currents in the cell, which is somewhat different for each cell.
To move beyond a basic phenomenological understanding of action potentials it is necessary to have a thorough understanding of voltage-gated ion channels, which are described in subsequent sections of this chapter.
Evolution of Electrical Excitability
A more general definition of electrical excitability is that when triggered the ion channels in the cell membrane follow a stereotypical path through state space before returning to their original state. Action potentials in most animal cells are mediated by changes in the state of the voltage-gated sodium and potassium channels. Electrical excitability in other species can have quite different mechanisms.
The voltage-gated and agonist activated ion channels that mediate electrical excitability in animal cells first evolved in prokaryotes. The basic functionality of these proteins remains largely unchanged in animals, although there is considerable expansion of gene families and specialization of function in animals.
In prokaryotes energy generation via chemiosmosis is performed by the cell membrane. This essential function likely constrained the evolution of other membrane functions such as electrical excitability, although ion channel mediated chemosensing and even voltage-induced calcium signaling have been described in prokaryotes.1 During the evolution of eukaryotes the cell membrane was freed from the role of energy generation, which was assumed by the mitochondrial membrane. This change probably facilitated the evolution of electrical excitability in early eukaryotes as well as more complex environmental sensing by specialized ion channels in the cell membrane.
Electrical excitability is a common feature in single cell eukaryotes. The increased sophistication of the electrical signaling and sensing function of the cell membrane in single cell eukaryotes contributed to a significant increase in behavioral complexity compared to prokaryotes. This includes the evolution of active predation of prokaryotes and even other eukaryotic cells. Remarkably, differences in the rate and pattern of action potential firing in some single cell eukaryotes can trigger different behaviors, foreshadowing a key feature of nervous system function.
A key advantage of electrical signaling over other signaling modalities is that electrical signaling is relatively fast. Predation and escape from predation creates strong selective pressure for rapid responses. In single cell eukaryotes action potential firing is often associated with the triggering of escape behaviors. Many of the subsequent advances in the efficiency of electrical function in the nervous system of multicellular animals are driven by a similar dynamic, an arms race between prey and predator.
References
1. Bruni, G. N., Weekley, R. A., Dodd, B. J. T. & Kralj, J. M. Voltage-gated calcium flux mediates Escherichia coli mechanosensation. PNAS 114, 9445–9450 (2017).